One vendor, one solution, all you need for thermal GxP compliance
One vendor, one solution, all you need for thermal GxP compliance
The platform that keeps global life science enterprises compliant – continuously







+1000 customers worldwide
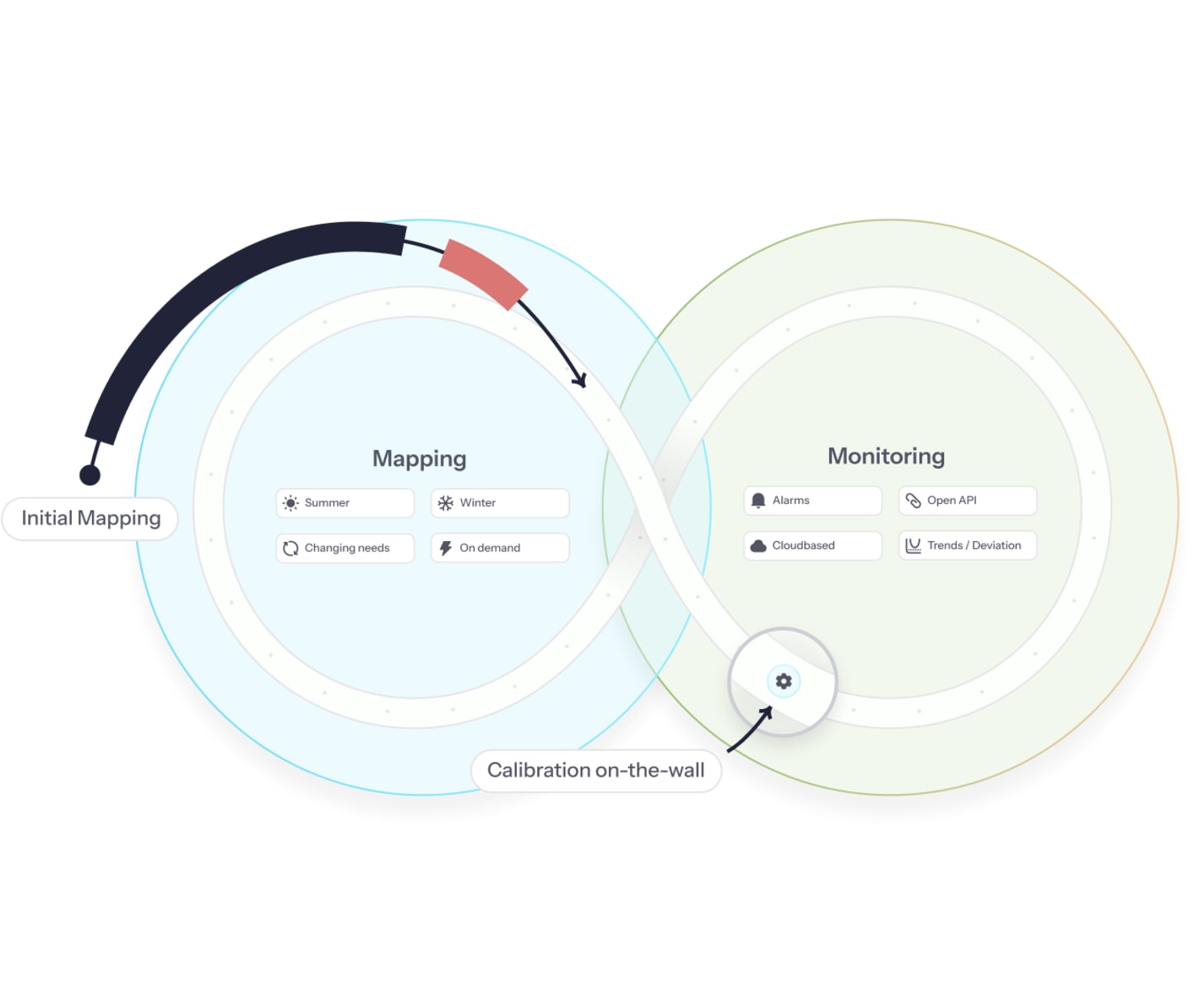
Mapping, monitoring, and calibration in one GxP-compliant solution

Validation
Conduct faster, more reliable validations in less time, at lower cost with the fastest mapping solutions and (on-site or remote) CQV services you will find.
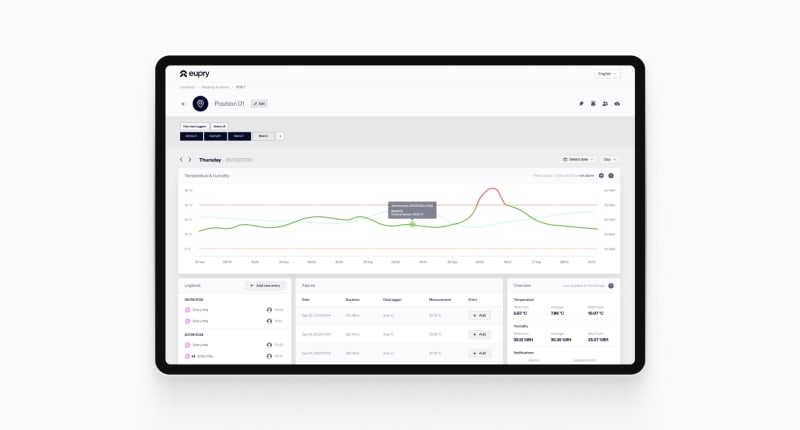
Monitoring
Free time and minimize risks with automated Wi-Fi-based monitoring, proactive alerts, and 3-click digital audit reports across sites, units, and departments.

Calibration
Reduce the time you spend on calibration by 95% with the included – automated and patented – calibration solution.
Global overview of all sites from anywhere in the world
On-the-wall calibration in 5 seconds
Old sensor out. New sensor in. Calibration is done.
Eupry’s patented solution redefines how calibration is done in GxP, letting you handle all your calibrations in minutes, directly at site, without changing data loggers.
= 95% less time spent on calibration.
All your data gathered in one platform
Monitoring. Alarms. Calibration certificates. Audit reports. Part 11 tracking. You name it. All available in the platform.

Download a product catalog
Get instant access to all the technical specifications, how the solutions work, and find an overview of all equipment in the Eupry catalog.
Trusted by +1.000 enterprises worldwide
See what our clients say
Managing thermal compliance across global operations

Turn compliance data into decisions
Track excursions, calibration cycles, and validation progress across all sites – and act on what the data shows you.
Automated quality tracking
Monitor excursions, calibration status, and validation progress across every facility from one dashboard.
Cross-site benchmarking
Compare sites to identify which have the most risks and need for quality resources.
Predictive control with AI
Get notified when patterns indicate equipment issues before they cause excursions.
No more re-mapping with continuous mapping and monitoring
- 25% lower total cost of ownership
- Fewer interruptions, lower workload
- Central quality control (of all sites)
- 6 x richer quality data foundation

Enterprise-grade cloud security built for GxP
Keep your pharmaceutical data secure and audit-ready while meeting global compliance standards.
Compliant by design
Hosted on AWS with ISO 27001, ISO 27018 and over 140 global standards met.
Full encryption, always
AES-256 for storage. TLS 1.3 for transfer. Encrypted backups across locations.
Strict access controls
Role-based access and zero-trust authentication down to device level.
Audit-ready architecture
Immutable logs, real-time monitoring and 24/7 incident response protocols.
Client Testimonials
Hardware built for GxP
Everything you need to monitor and validate your pharmaceutical facilities and units.

Wireless data logger
Wireless temperature monitoring with 2-year battery life and secure, encrypted data transfer

External Temperature Sensor
Ultra-wide range digital temperature sensor from -100°C to +100°C with silicon bandgap technology.

External Temperature & Humidity Sensor
Compact digital sensor for temperature and humidity monitoring in ambient and controlled environments
Ready to get started?
Discover how Eupry's automated compliance (monitoring, calibration, and validation) solutions work today.











