Pharmaceutical warehouse compliance overview

Jakob Konradsen
Understand pharmaceutical warehouse compliance for GDP/GMP – from validation, temperature mapping, and monitoring to calibration and documentation. Learn what regulators expect and how to keep your warehouse consistently compliant for pharmaceutical storage.
Get an actionable 13-step list with all the steps you need for GxP compliance.

Pharmaceutical warehouses are critical in maintaining the safety of medicines, biologics, and vaccines before they reach patients. To comply with GDP and GMP, warehouses must demonstrate strict control over storage conditions. This involves validation, mapping, monitoring, calibration, and thorough documentation.
This page provides a complete overview of pharmaceutical warehouse compliance, referencing GDP, GMP, and ICH guidelines. It connects you directly to detailed guides, checklists, and solutions so you can act with confidence and be audit-ready.
What is a pharmaceutical warehouse?
A pharmaceutical warehouse is a controlled storage facility for medicines, vaccines, and biologics. Regulators such as the EMA, FDA, and WHO expect warehouses to maintain controlled temperature and humidity ranges, supported by documented studies and monitoring systems. Compliance ensures product quality, patient safety, and business continuity.
What are the main compliance requirements for warehouses?
To comply with Good Distribution Practice (GDP) and GMP standards, warehouses must:
- Environmental control: maintain both temperature and humidity within defined ranges, supported by validation and monitoring.
- Mapping before use: perform temperature mapping to identify hot/cold spots and validate suitability. See the warehouse mapping guide.
- Continuous monitoring: implement monitoring based on mapping outcomes, with alarms and corrective action processes. Explore the warehouse monitoring guide.
- Requalification: re-map when facilities, equipment, or layouts change, or at regular risk-based intervals. Learn about warehouse mapping services.
- Documentation: provide audit-ready digital records, calibration certificates, deviation logs, and alarm histories. See the warehouse monitoring solution.
Tip! Check the GDP warehouse compliance checklist for a step-by-step overview.
Who is responsible for warehouse compliance?
Compliance in pharmaceutical warehouses can involve multiple roles. Heads of Quality and QA Managers are often responsible for setting compliance strategies and budgets. Validation Engineers design and run mapping and qualification studies. Local Facility Managers ensure daily operations align with requirements, even though compliance may not be their main expertise. Together, these roles form the foundation of audit readiness and regulatory confidence.
How do you validate a pharmaceutical warehouse (IQ/OQ/PQ)?
Validation follows international standards (ICH Q1A, GMP, GDP) and typically includes:
- IQ (installation qualification): verify that sensors, equipment, and software are installed as designed.
- OQ (operational qualification): confirm the system performs within defined ranges under controlled conditions.
- PQ (performance qualification): demonstrate consistent control in real-life conditions, including worst-case scenarios.
Read more in the IQ/OQ/PQ for warehouses guide.


Compliance checklist for pharmaceutical warehouses
Download a free temperatue compliance checklist for pharma warehouse. Get a step‑by‑step guide to help you qualify warehouses, execute mapping, set up monitoring, and document results in line with GDP expectations.
What is temperature mapping in warehouses?
Temperature mapping establishes a baseline for compliance by measuring distribution of conditions across the warehouse.
- Protocol-driven approach: develop a mapping protocol defining placement, duration, and acceptance criteria.
- Study length: typically 7–14 days to capture normal operations.
- Seasonal studies: perform in both summer and winter to account for extremes, as required by GDP.
- Reporting: document findings and corrective actions for future monitoring setup.
Tip! Looking for a mapping service or equipment? See how Eupry's GxP-tailored warehouse mapping services work.

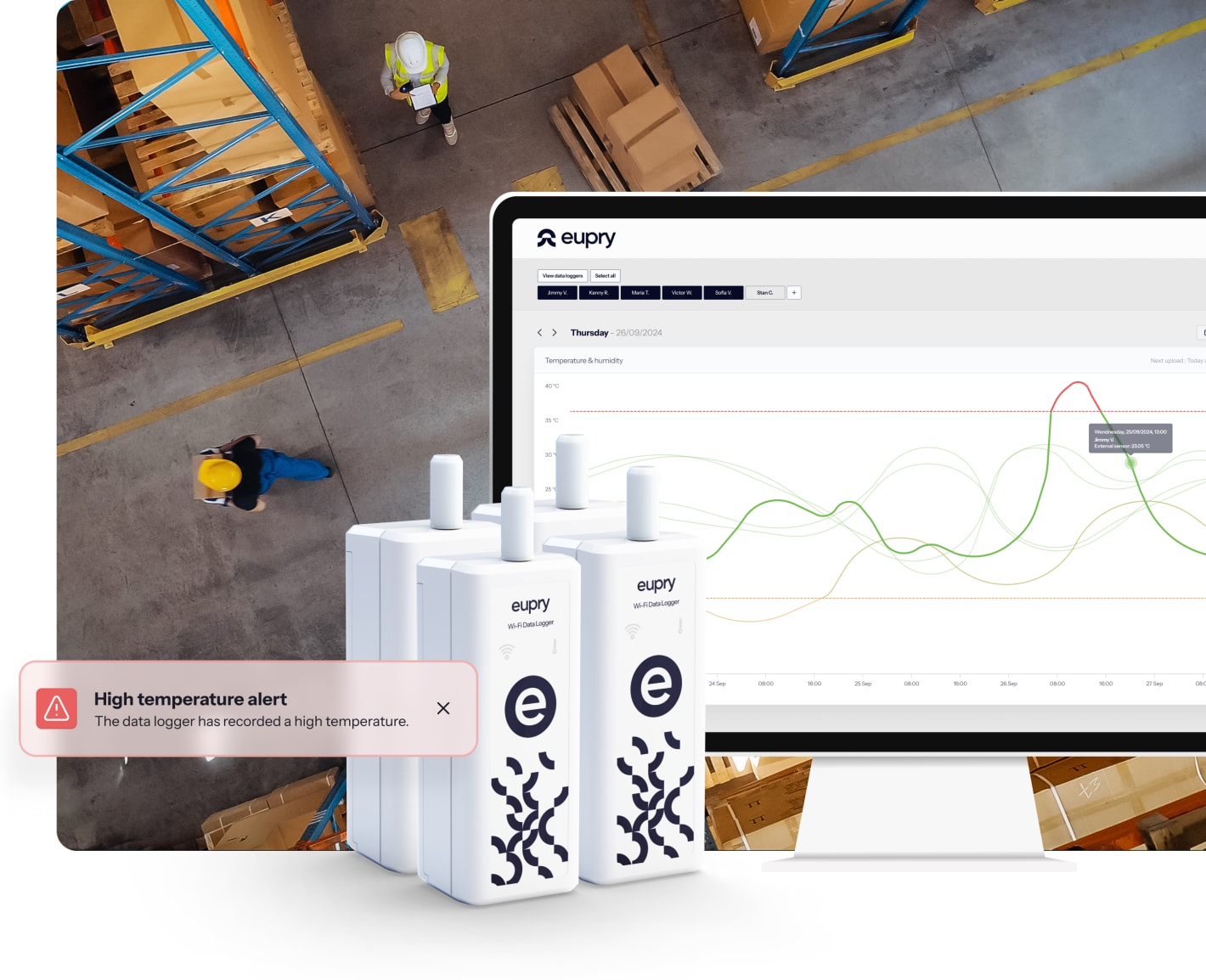
How should warehouse temperature monitoring be set up?
Monitoring systems must reflect risks identified during mapping. A compliant setup includes:
- Sensor placement: at hot/cold spots, entrances, shelving, and other risk-prone areas.
- Alarm strategy: defined escalation paths, thresholds, and corrective action timelines.
- Periodic review: documented checks of records, trends, and deviations.
- Digital records: secure, retrievable data for audits, preferably linked with calibration certificates.
See how Eupry's automated warehouse monitoring solution works.
Why are calibration and documentation important for warehouses?
GDP and GMP require use of calibrated equipment traceable to ISO 17025 standards. Without valid calibration, monitoring data is not acceptable for audits or regulatory submissions.
A best-practice setup integrates:
- Accredited calibration of all loggers and sensors
- Automatic linking of calibration certificates to monitoring records
- Secure audit trail covering deviations, alarms, and corrective actions
What is continuous temperature mapping in warehouses?
Continuous temperature mapping is an emerging framework recognized in GxP guidance and discussed by ISPE as "continuous verification." Instead of repeating disruptive re-mapping studies every 1–3 years, warehouses place a larger network of calibrated sensors that continuously generate data robust enough to meet both mapping and monitoring requirements.
This approach provides:
- Ongoing compliance evidence without repeating periodic studies
- Richer data across more points, strengthening risk assessments
- Centralized quality control across multiple facilities
For pharmaceutical warehouses, continuous mapping can reduce interruptions, minimize compliance risks, and create a stronger audit trail. While still subject to risk assessment, it is increasingly seen as a best practice for large or complex facilities.
FAQ questions about pharmaceutical warehouse compliance
Digital temperature compliance for pharma warehouses
One solution, all you need for thermal compliance
Eupry brings temperature compliance – mapping, monitoring and calibration – into one digital, GxP-compliant solution.
Ensure compliance of your pharma warehouse and any other facility and gain quality control across sites in less time, at a lower cost, only using one vendor.
- Full digital overview and audit reports in 3 clicks
- Automated monitoring in real-time
- On-site and remote mapping solutions built for GxP
- The fastest calibration on the market
How Eupry's solutions enables warehouse compliance
Warehouses present one of the toughest compliance challenges in pharma – multiple zones, seasonal changes, and high-value stock. Eupry turns this complexity into a clear, controlled process.
- Scalable mapping: From 15 to 300+ loggers, our quality team helps design the right grid for your floor plan and storage conditions.
- Central quality control: See compliance across every warehouse worldwide in one dashboard – from Copenhagen to California.
- Continuous mapping: Replace disruptive re-mappings with a setup that provides constant GDP-compliant validation.
- Unified compliance: One solution for mapping, monitoring, and calibration – no more managing multiple vendors.
- Smarter identification: Consistent naming conventions make finding loggers easy across large facilities.
3 reasons why it works:
- Facility-specific recommendations
- Simple logger identification
- Reliable compliance at global scale
Get an instant overview of how the monitoring, mapping, and calibration solutions work and get all the technical specifications.