Continuous temperature mapping (and monitoring)
No more re-mappings. Constant compliance. Less costs.
Make sure that your entire operation continuously meets GxP compliance requirements with continuous temperature mapping.
- Reduced total cost of ownership
- Fewer interruptions, lower workload
- Central quality control (of all facilities)
Instant access to all technical specifications and more.
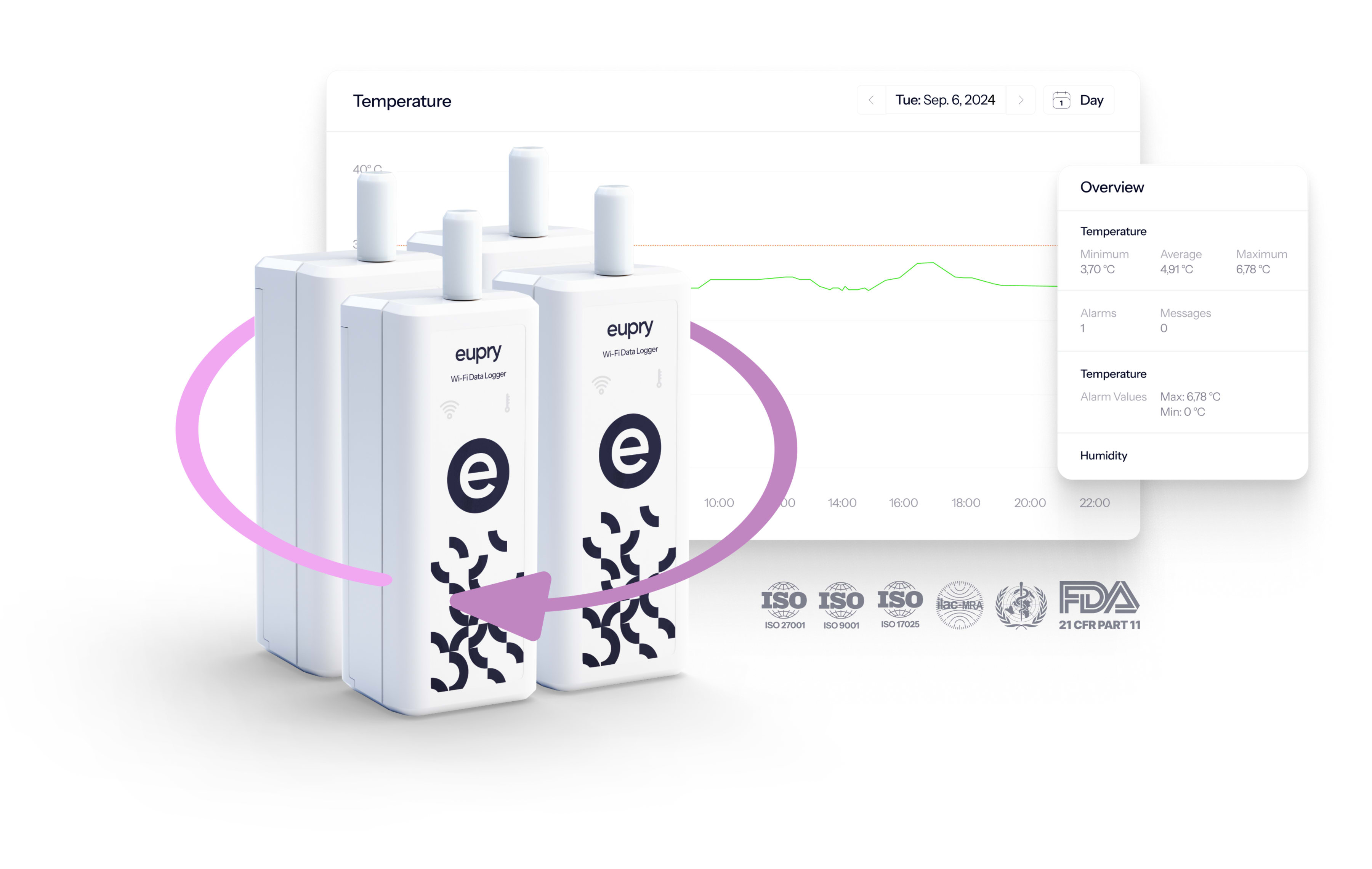
One solution keeping your operation – continuously – compliant
Eupry’s Continuous Temperature Mapping Solution covers all your validation, monitoring, and calibration requirements – in less time and at a lower cost.
The solution provides:
- Constant compliance: The setup consistently provides data sufficient to cover both validation and monitoring requirements .
- No more re-mapping: This eliminates the need to conduct – and keep track of – re-validations.
- Central quality visibility: With the online platform, you can easily monitor quality across all your facilities, units, and departments.
In other words, your operation is – and remains – compliant.
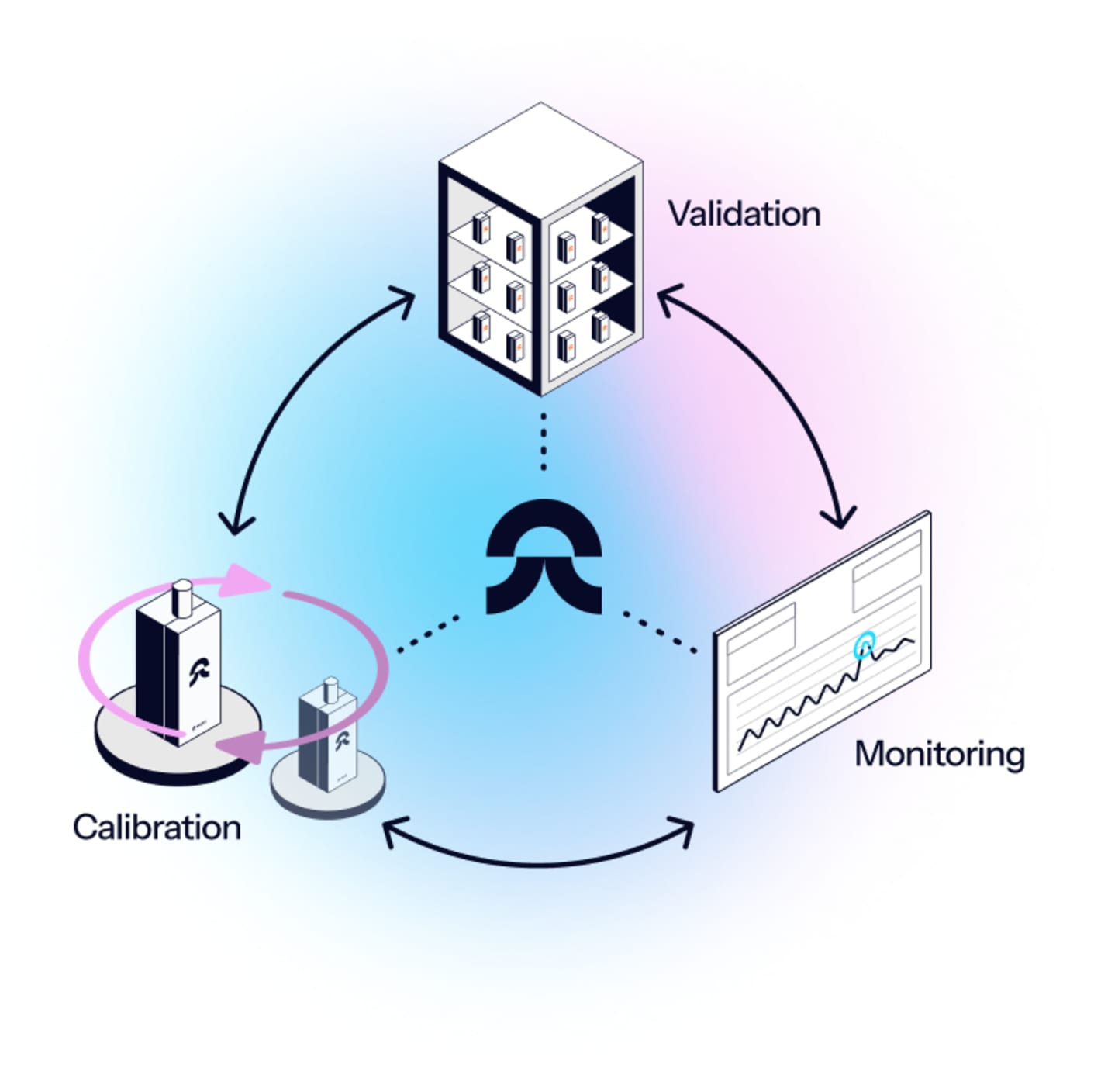

= No more re-mapping
Eupry’s Continuous Mapping Solution eliminates the need for re-validation (unless the operation changes), meaning you no longer need to worry about keeping up with validation schedules across sites.

One validation – and you are done!
No re-mappings
No unnoticed quality risks
No hunting for information
No critical validation needs
The components of the continuous mapping solution
Tailored risk assessment
Continuous mapping is built on a risk-based approach. Therefore, it begins with a risk assessment conducted by our validation team, which lays the foundation for designing a solution for your operation and provides documentation for auditors to prove that all requirements are being met.

Wireless data loggers
With the wireless Eupry data loggers, you have all the information you need at your fingertips. The loggers automatically transfer data to the software and send instant alerts in case of deviations – also, calibration is included.

Temperature compliance platform
The included platform gathers all documentation, providing an overview of validation results, monitoring records, and calibration certificates. It is easy to use (also for teams outside the quality field) and lets you monitor compliance from any screen, automate tasks, tailor deviation alerts, design validations, analyze data, and generate audit reports in seconds.

Specialized support
Temperature compliance is what we do. With hundreds of mappings and monitoring setups under our belt, we are on top of the requirements of GxP to make sure you become – and stay – compliant. Our team tailors a setup for you, and we are always just a call away when you need us.

Add-ons (if you need them)
- Mapping services: On-site, remote, or DIY mapping options tailored to meet GxP standards.
- Centralized quality dashboard: One dashboard that gives a complete overview of compliance for all locations.
- Standardized processes: Our team can help turn your requirements into actionable SOPs to harmonize procedures – and compliance – in your operation.


“I have nothing but praise for Eupry. They have invented a system that is simple and does everything it promises. Loggers are reliable and the user interface is logical, intuitive, and detailed in a way so I can easily monitor and pull out reports.”

Allan Witt
GDP Responsible Person at Worldwide Flight Service

“The calibration of the Eupry temperature loggers is easy and quick. I received a box with the new sensors (…) It took me under an hour to calibrate 40 loggers – including the time it took to find the refrigerators.”

Kasper H. Christophersen
Research Associate at Novozymes
Reduced costs
Trim costs by over 30% and reduce your compliance workload by minimizing validation work and automating manual tasks.
Constant compliance
Guarantee continuous compliance across the entire operation. Never worry about validation deadlines, unnoticed compliance issues, or audit surprises again.
Fewer disruptions
Reduce the burden on teams with way (!) fewer validation interruptions, standardized procedures, and an automated solution.
Higher quality
Showcase top-tier quality standards to clients. Temperature compliance will no longer stand in the way of – but support – your business growth.
All you need for thermal compliance
Minimize the amount of suppliers in your life. Eupry’s Continuous Mapping Solution covers both mapping, monitoring, and calibration.
Better data, full visibility, total quality control
Integrating monitoring, mapping, and calibration in one digital platform creates better data than ever. Get the insights you need to gain full control of quality across your operation.
A digital overview of the compliance level of all sites means no need to travel or dig through piles of documentation.
- Make sure that all sites continuously meet your requirements
- Conduct trend analysis and compare KPIs across facilities
- Mitigate risks before they evolve and make proactive improvements

Complete compliance confidence
Minimize the risk of non-compliance by making sure your operation consistently meets validation requirements. Also, our digital compliance solutions, of course, check all the requirement boxes of GxP:
- FDA 21 CFR Part 11 module
- ISO 17025-accredited calibration
- ISO 9001-certified quality management
- High compliance rating by, among others, Novo Nordisk
- Fully IT and hardware-validated

Minimized friction when scaling your business
New locations? New product types? With quickly deployable validation services, extensive sensor options, wireless data loggers, and a cloud-based system, expanding your operation is just a call away.

FAQ about continuous temperature mapping
The often asked questions and their answers about the continuous mapping method.

Download your free product catalog
See how the continuous mapping solution and works in the catalog, and get an overview you can automate and collect monitoring, calibration and mapping in one GxP-ready, digital solution.
Get started with continuous mapping
Need a quote or more information? We are here to help. Have a talk with one of our specialists - or see how it works in our catalog.
