Uncomplicate your temperature mappings
- Avoid costly delays
- Validation experts on call
- Easy digital reporting


Simplify your mapping process. No worries, full compliance.
Downtime, errors, unnoticed failures, equipment variety: Temperature mappings are complex.
Eliminate complications and gain solid confidence in your mapping study’s quality – no matter the equipment type, number, and size.
Legal basis and guidance
The mapping solutions are designed with a legal basis in cGMP, USP <1079>, and EudraLex Volume 4 Part 1 Chapter 3, and based on guidance from WHO guidelines (supplement 8 and 7 and annex 8), DKD-R 5, FD X 15-140 (French standard), and the ISPE Standard “Controlled Temperature Chamber Mapping and Monitoring”.
Client Testimonials

Get your free product catalog
See all the technical details about how the temperature compliance solutions work.
What are the benefits?
Process transparency
Get a complete, digital overview of every step of the mapping process. Control the process to control compliance.
No calibration blocks
Pre-calibrated data loggers and automatically linked certificates in the software mean no manual pairings – and no waiting time.
Live monitoring
Live monitoring lets you catch issues right away, minimizing the risk of operational holdups. No costly delays.
Compliance certainty
ISO 17025-calibrated, ISO-9001-certified, and FDA 21 CFR Part 11-simplified – rest easy, you are fully compliant.
Efficient reporting
Minimize time spent on reporting while securing a more reliable outcome through automated data collection and analysis.
Mapping expertise
No need to know the details of ever-changing product recuirements and regulations for mapping various equipment – we have got you covered.
Skip unneeded costs
No more purchasing hardware for occasional studies just to see it collecting dust. The leasing-based model gives you access to equipment when you need it.
Someone to call
Have questions along the way? Our validation specialist will be there to guide you through the process.
Temperature mapping that just works
Go hands-on with a leased GxP-compliant mapping kit designed to perform quick and reliable studies in highly regulated industries like pharma, biotech, and logistics – or let our validation team take care of the study for you.
How the full mapping service works
From protocol to on-site conduction of the study and finalized report: Our team of experienced validation experts handles every step of the way.
The short version:
- You tell us what you need (or we figure it out together)
- We create the protocol and you sign off
- We conduct the study*
- We analyze the results
- We create the report
- You use it to make auditors happy and improve processes
- We train your team to do it all in the future (optional)
Tip: The mapping service can also be utilized in a remote version. Our experts still take care of everything from protocol to the final report – you simply place the data loggers yourself.

Tailored for your conditions
From freezers and fridges to warehouses and containers: Regardless of the type, number, and size of equipment, the solution accommodates them and adapts to any scale.
The solutions also apply to both:
- Operational qualification (OQ): Typically, empty room studies to ensure that the equipment operates to the needed standard.
- Performance qualification (PQ): To validate equipment function, we simulate real-world conditions with full rooms or “dummy packages”. This also includes testing for power failures and door openings.
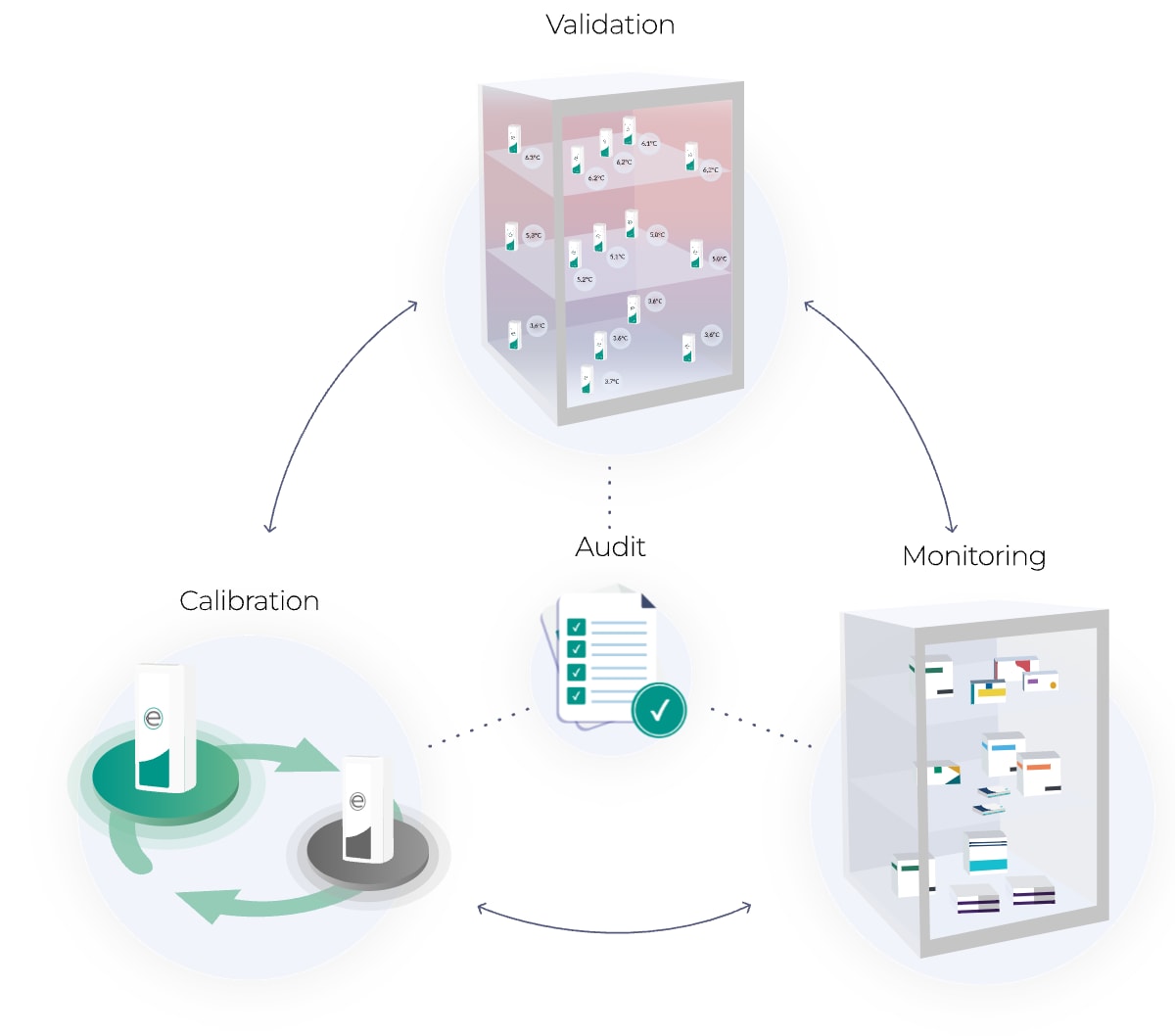
Much more than temperature mapping
Eupry is not just about mapping. It is about removing the mess from all temperature compliance.
It is a digital ecosystem that collects all your temperature compliance efforts into one solution – from temperature monitoring to data logger calibration and mappings – removing the messiness of your processes and the need for several providers.

