Autoclave validation services
From qualification to continuous GMP/GLP compliance
Eupry offers pharmaceutical autoclave validation without the multi-vendor chaos, long timelines, or surprise costs.
- Full IQ/OQ/PQ coverage
- Digital protocols built for GMP & GLP
- One vendor, all thermal compliance
Fill out our contact form to get more information or set up a talk with a specialist.
Trusted by 1000+ companies worldwide
Long timelines, disruptions, disconnected compliance
Validation blocks production
Traditional autoclave validation often requires weeks of downtime, creating scheduling issues and months of production lost.
Requalification restarts
Every year (or after changes), the whole process starts over: vendor scheduling, downtime, costs, and manual reporting.
Disconnected validation data
Validation produces paper work, every cycle requires new manual entries, and auditor questions result in digging through records.
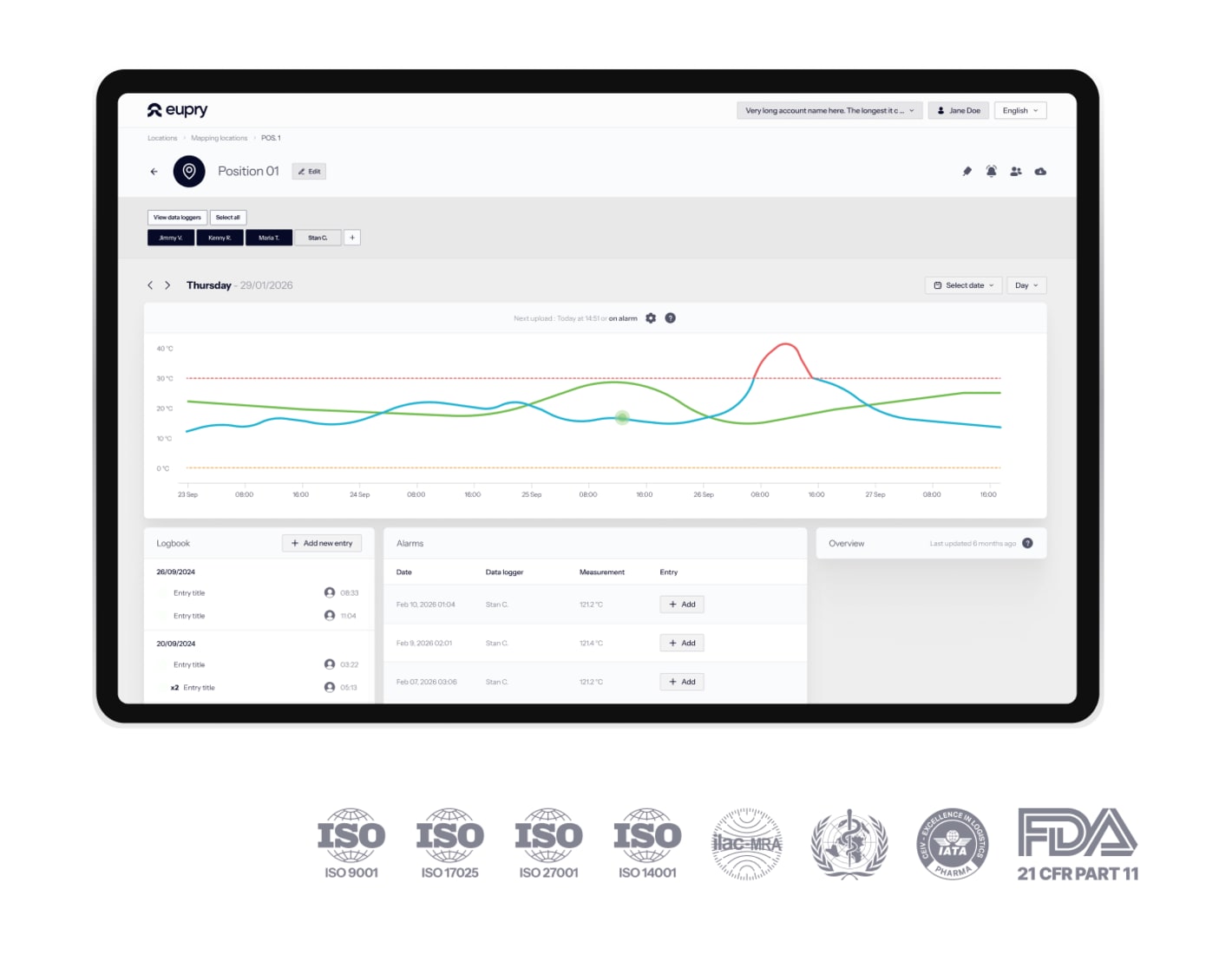
Faster validation, continuous GMP and GLP compliance
Minimize equipment downtime and connect your validation data directly to ongoing cycle monitoring with Eupry.
Reduced equipment downtime
With efficient wireless equipment designed for GMP.
From validation to monitoring
One trail and solution for the entire unit lifecycle.
Automated and digital validation
Zero manual logging with automated digital records.
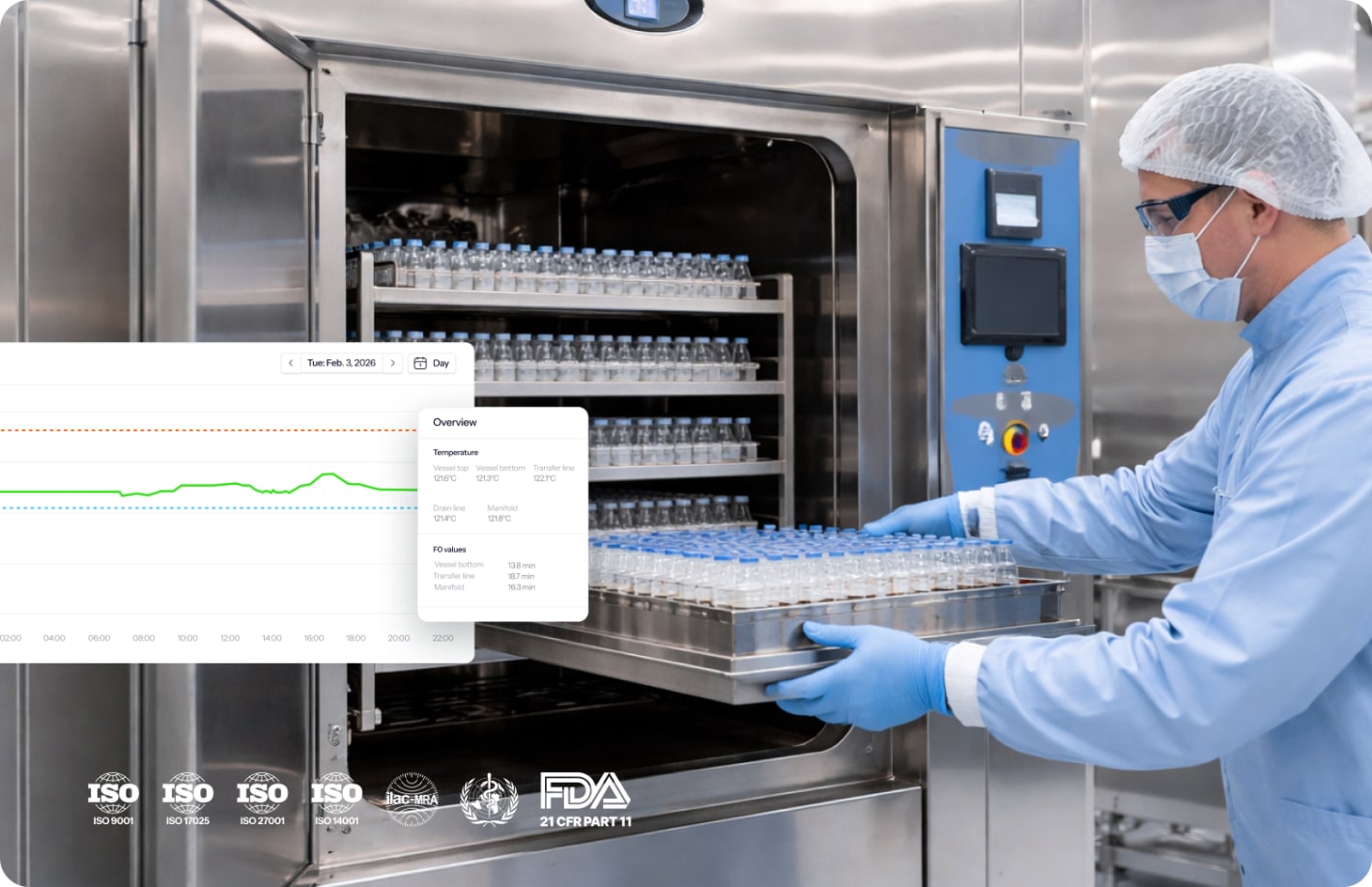
No more manual work with automated validation
The pre-calibrated Wi-Fi sensors transmit validation data in real-time.
- No manual data transcription or Excel entry
- Automated F₀ calculations during PQ studies
- Live monitoring during validation runs
- Digital protocols and 3-click reporting


Talk to a specialist
Need more information or a tailored quote? Fill out the form, and we will be in touch as soon as possible.
Why choose Eupry for autoclave validation?
Validation expertise you can trust
Our validation engineers have conducted hundreds of qualifications across pharma, biotech, and other GxP industries and understand the regulatory expectations from FDA, EMA, and MHRA inspectors.
Digital protocols auditors prefer
Our digital validation system provides complete data capture. Eevery reading is timestamped and attributed, calibration certificates are embedded, and report can be generated digitally.
Complete compliance documentation
Every validation includes ISO/IEC 17025 calibration with NIST traceability, ISO 17665:2024 and EN 285 compliant protocols, 21 CFR Part 11 ready platform, and GMP aligned qualification approach.
One vendor for all your units and facilities
At Eupry, we handle validation and overall thermal compliance for any temperature-controlled unit or facility in GxP - from your autoclaves, fridges, and freezers to warehouses, cold rooms, and air crafts.
Complete IQ/OQ/PQ equipment validation of autoclaves
Eupry's autoclave validation services include:

Installation qualification (IQ)
We verify correct installation per manufacturer specifications, confirm utility requirements, test safety system functionality, and document ISO/IEC 17025 calibrated instrumentation – all with digital checklists and photo documentation.
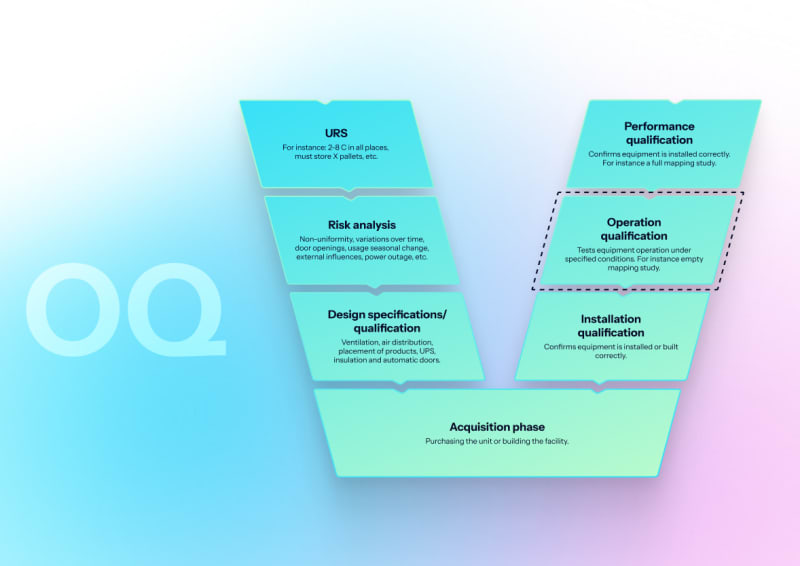
Operational qualification (OQ)
Empty chamber validation with vacuum leak testing with documented pressure rise rates, Bowie-Dick tests, multi-point heat distribution mapping, EN 285 steam quality verification (dryness, NCG, superheat), and control system and alarm challenge testing.
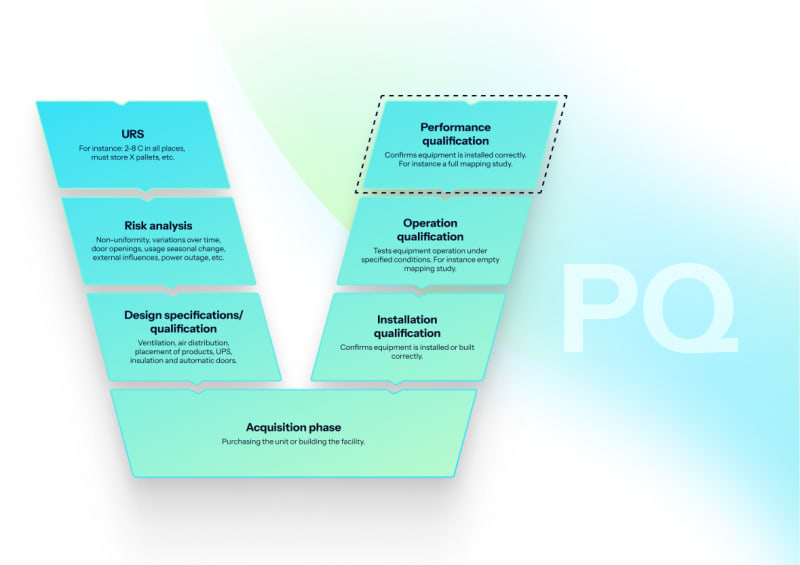
Performance qualification (PQ)
Loaded chamber validation with worst-case load determination, heat penetration studies, automated F₀ calculations, biological indicator challenges (G. stearothermophilus, 10⁶ CFU), and load dryness verification for porous loads.
End-to-end biological indicator management
Eupry's team manage the complete BI challenge process to provide a full package, proving sterility assurance level achievement.
- Source certified G. stearothermophilus spore strips
- Strategic placement in worst-case load locations
- Post-cycle incubation at our facility or yours (7 days at 55-60°C)
- Daily growth monitoring with photographic documentation
- Growth/no-growth certification with BI lot certificates


Download a product catalog
Get an easy overview of all Eupry’s products from validation to calibration and automated monitoring temperatures, humidity, CO2 and more.
One vendor, one solution, all you need for thermal compliance
Collect validation, monitoring, and calibration in one GxP-compliant solution.
- Up to 25% lower total cost of ownership
- Standardized processes across sites
- One source of truth for all thermal compliance data
- 3-click audit reports
Get instant access to an easy overview of how the solutions work and the technical specifications.






