Cold room monitoring system for GDP and GMP
Cold room monitoring made simple. Keep your pharma facilities compliant and under control – without manual work.
- Instant SMS/e-mail alerts: Stop deviations before they cause issues.
- 3-click audit reports: GxP/ISO 17025/Part 11 documentation in clicks.
- Central control: Get one digital overview across sites and units.
- Reliable sensors: Built for cold rooms and ultra-low environments.
Get instant access to all solution options, technical specifications, and more.

Trusted by +500 pharma and healthcare logistics companies worldwide including:
Automated monitoring of cold rooms
Anywhere, on any screen, without manual work
Eupry's monitoring solutions are tailored to GxP - automate your monitoring practice across all rooms and other temperature-controlled facilities and TCUs.

Instant alerts
Get notified immediately when the temperatures of your cold room or other facility move out of range, with smart alarm filtering and custom alerts by SMS and e-mail.
Wireless data loggers
The Wi-Fi-based data loggers - built for GMP and GDP - send data automatically and are come with custom sensors for any cold (or ambient) storage environments.
3-click audit report
Track compliance on any screen – from desktop to phone – and generate full reports in the cloud-based platform containing all the data you need to secure (and prove) GxP compliance.
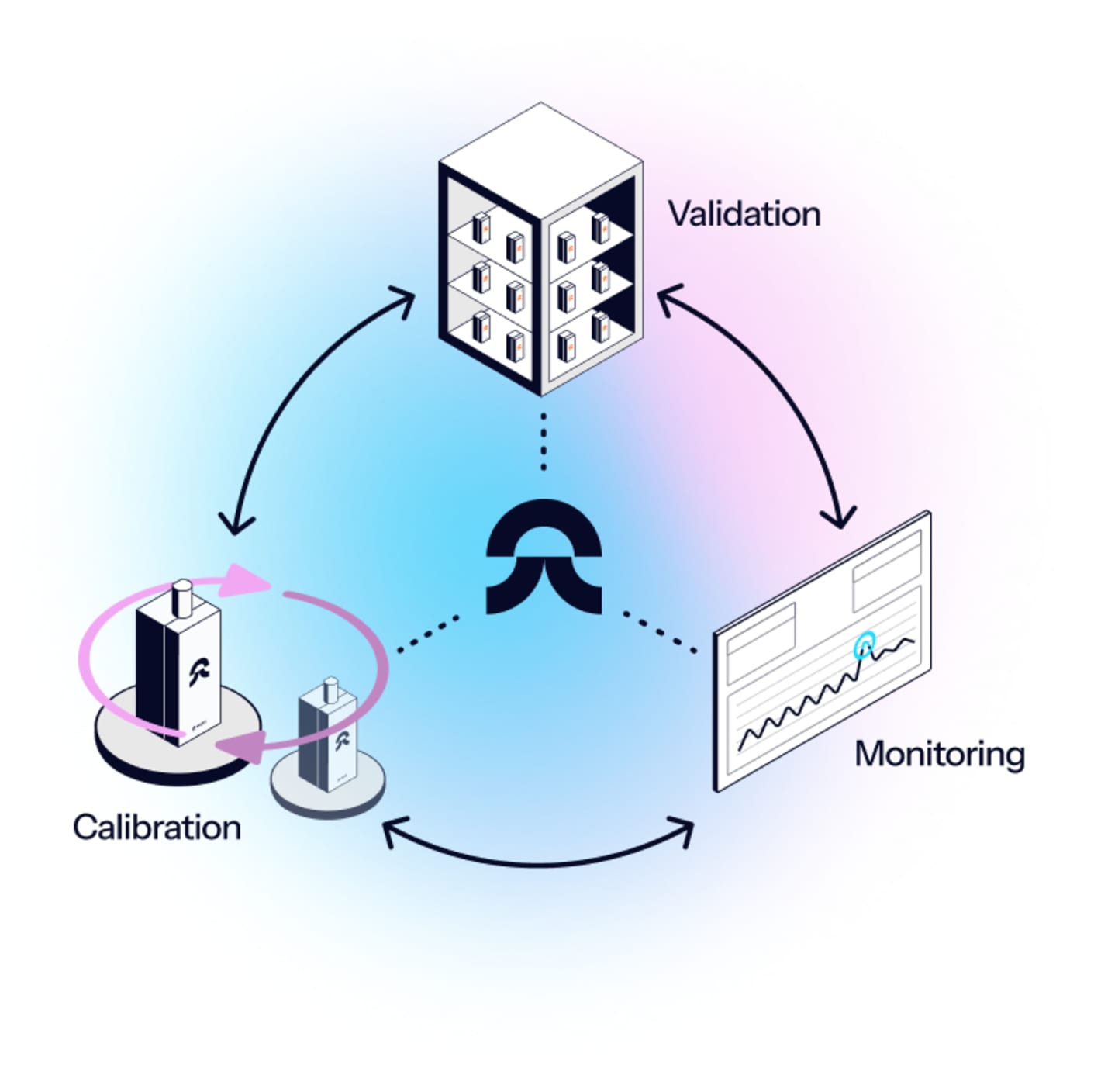
Central control
Get one digital overview of the quality of your entire operation – and monitor all facilities, TCUs, and sites, from one dashboard, with validated backups to protect data during connectivity issues.


Download your free product catalog
See how the cold storage monitoring solution works, get access to all technical specifications, and find all product options in our free catalog.
Cold room monitoring technology

Wireless Wi-Fi loggers
The Wi-Fi-based data loggers automatically send data through Wi-Fi to your platform to let you monitor conditions live and without manual transfers.

Sensors tailored for cold room conditions
Choose between sensors for any temperature and humidity monitoring needs within GxP. All sensors can be used for both monitoring and mapping.
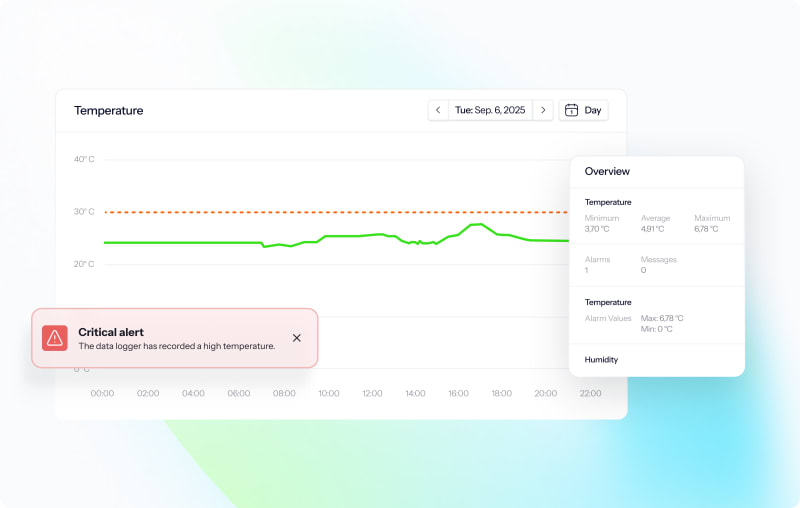
Monitoring platform
Access everything you need to secure and prove compliance: audit reports, monitoring data, deviation logs, calibration certificates, and more – all historical data, just a few clicks away.
The fastest calibration you will find included
Handle all your calibrations n-site, in seconds, without changing data loggers.
Eupry's on-the-wall calibration solution changes how we handle calibration in GxP - plus, you will get automatic notifications when calibration is due. No more calibration worries or disruptions to your operation.

Why choose a cold room temperature monitoring system from Eupry?
Stay compliant, always
Be in full control of compliance at all times and showcase top-tier quality with live monitoring, instant alerts, and no manual errors, process gaps, or missed quality issues.
Free up (a lot of) time
Fewer disruptions, smaller workload, no paperwork. Spend way (!) less time on compliance with automation and one easy-to-use digital solution gathering all temperature compliance.
Minimize risks & costs
Cut overall costs and avoid surprise fees with a unified, cost-optimized solution that includes calibration and a full warranty. No hardware issues, no replacement costs.
All your compliance boxes?
Consider them ticked!
At Eupry, compliance is at the forefront of everything we do. We keep up with every regulatory turn to keep the solution – and you – a step ahead.
- GDP & GMP monitoring standards
- ISO 17025 accredited calibration
- FDA 21 CFR Part 11 module for records/signatures
- ISO 9001-certified quality
- ISO 27001‑certified data security
- IQ/OQ Qualification protocol

Automated temperature monitoring for cold rooms
.. and any other GxP-regulated storage environment.
Get instant access to all the technical specifications, solution options, and more in the free product catalog.

One vendor, one solution, all you need for thermal compliance
Ensure compliance and gain quality control across sites in less time, at a lower cost. Eupry brings all your temperature compliance:
- mapping
- monitoring
- calibration
into one digital, GxP-compliant solution.
Get an instant overview of all solution options and how they work.