IQ, OQ, and PQ services for GMP and GDP
One-stop for your entire validation process
Get your validations done faster with Eupry’s temperature qualification services built for GMP and GDP.
- One provider for the full validation
- Cost- and time-optimized process
- Full GxP compliance confidence

Get an instant overview of all the solution options, how they work, as well as all the technical specifications.
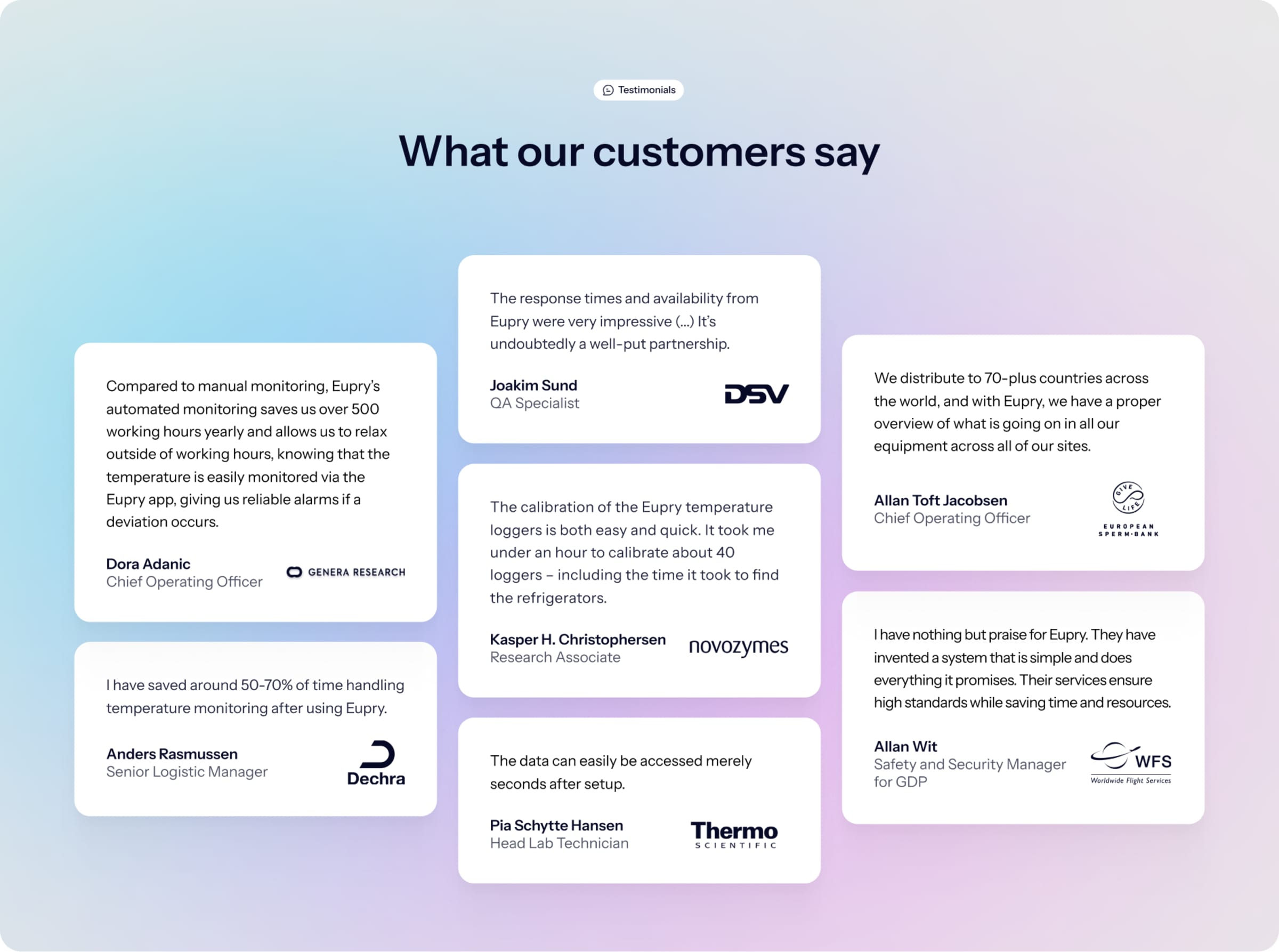
Trusted by +500 pharma and healthcare logistics companies worldwide including:
One provider, everything you need
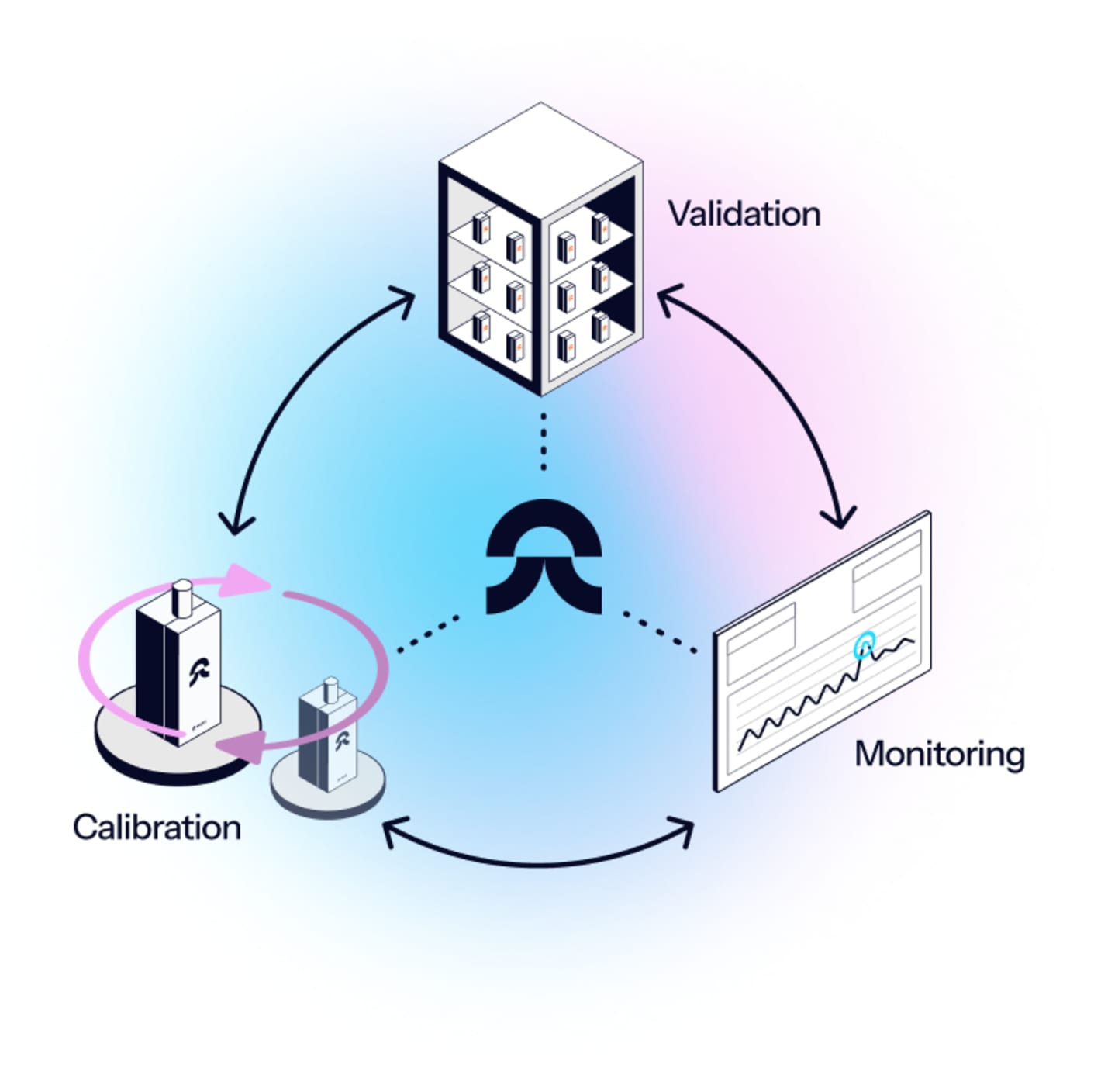
Eupry’s validation services cover the full qualification cycle: Installation qualification (IQ) to operational qualification (OQ) and performance qualification (PQ).
Let us handle the complete qualification of your fridges, freezers, incubators, or any other temperature-controlled units.
… or pick and choose the parts that make sense for your operations.
See all the solution options instantly.
Why work with Eupry for IQ, OQ, and PQ?
- GxP compliance confidence: GxP compliance is what we do, and our validation specialists can handle the full process, giving you peace of mind that your new CTUs or facilities are installed and operating correctly right from the start.
- One place for temperature compliance: From the initial IQ to the final PQ and continuous monitroing, our specialized GxP solutions cover all you need for thermal compliance.
- Minimized costs and time-spent: Cut overall costs and avoid surprises with a unified, cost-optimized, and automated solution that includes calibration and a full warranty.


Download a free IQ/OQ/PQ solution catalog
Ensure reliable temperature validation for GDP and GMP - in less time. See how it works in the free catalog.
Specialized in (pharma-level) temperature validation
Temperature compliance is what we do. With hundreds of validations under our belt, we know how to efficiently ensure your temperature-controlled equipment meets GMP, GDP, and other GxP standards and is ready for operation.
We have built flexible services and equipment (data loggers, sensors, and specialized software), all designed especially for temperature compliance in GxP.
= GxP-reliability, faster
Meet the standards of GMP, GDP, and other GxP regulations and take your units into operation – in less time – by centralizing the validation and qualification process with one specialized provider (us!).

Short version: What is the solution about?
- What it is: One specialized service covering the entire temperature validation process from installation qualification (IQ) to operational qualification (OQ) and performance qualification (PQ).
- Why it makes sense: To make the process as reliable and efficient as possible.
- Who it is for: Highly regulated industries like pharma, biotech, and pharmaceutical logistics.
- Which units it covers: Any temperature-controlled unit – from fridges, freezers, and incubators to cold rooms and warehouses.

How the services work
Our validation team handles the full IQ/OQ/PQ process of your temperature-controlled system.
Depending on your requirements, the services can include:
- Installation qualification (IQ): Our team verifies that the unit is installed correctly according to the manufacturer’s specifications.
- Operational qualification (OQ): We ensure that the unit meets all URS requirements, such as maintaining the required conditions and triggering alarms. The operational testing often includes empty temperature mapping studies.
- Performance qualification (PQ): This process validates the unit’s performance under actual working conditions, often through loaded mapping studies.
Tip! The mapping studies can be tailored to fit your specific needs – from our full-service option, where our team handles everything, to a more hands-on approach using our mapping kit. Either way, you will have our team’s guidance every step of the way.
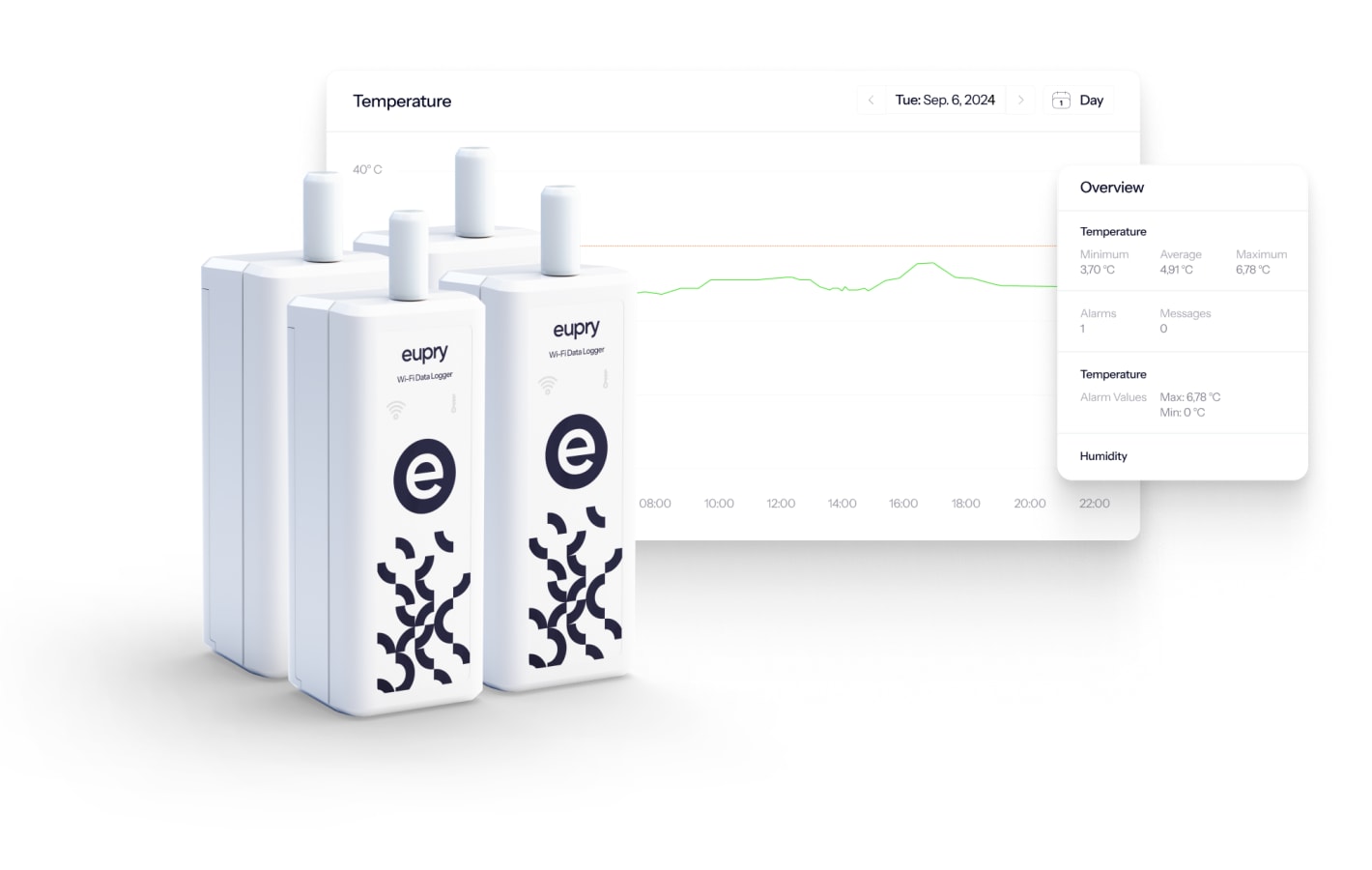
The fastest calibration solution you will find included
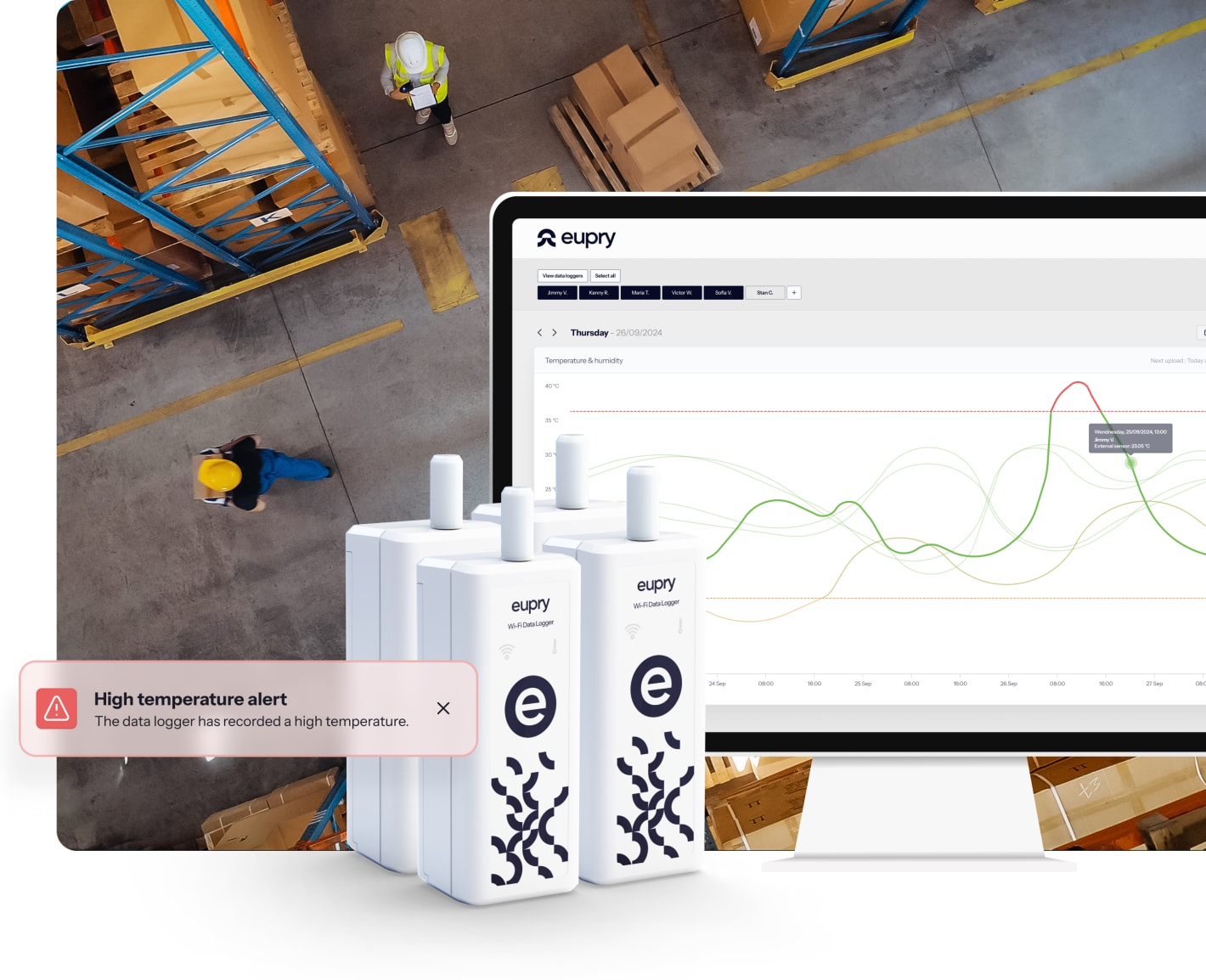
With Eupry's on-the-wall calibration solution, you can handle all calibrations on-site and in minutes, and thereby, reduce the time you spend on calibration by up to 95%.
Plus, data loggers are always pre-calibrated, and certificates are linked in the software, meaning no need for manual pairings and no idle time waiting for calibrations.

Turn temperature validation into your competitive edge
- Full GxP compliance confidence
- The most cost-efficient solution
- Minimized operational downtime
Taking new equipment into operation is a need to have when scaling your operations. With Eupry, temperature validation will go from being an operational roadblock to becoming your competitive advantage. Allowing you to quickly take your new unit into use.
Dependable compliance: GxP is our specialty, and as regulations evolve, so does our service. An experienced engineer handles your full project, and we proactively update our processes to meet the latest FDA and EMEA standards, ensuring that the solutions – and you – remain compliant.
Efficient compliance: With our well-tried processes and one provider for the full validation, you can get your equipment up and running faster without interruptions. With a few clicks in the software, you can transfer from validation to ongoing monitoring and avoid the added resources of managing multiple vendors, leading to lower overall costs.

Everything you need for temperature compliance
What about after validation?
We have got you covered. After the process is complete, our automated temperature compliance solution allows you to easily transfer to ongoing monitoring with just a few clicks.
And (the market’s most efficient) calibration is included.
Once the validation is done, you simply:
- remove the extra data loggers.
- switch from mapping to monitoring software with a few clicks.
And you are done.