Complete guide to steam-in-place (SIP) validation in GxP

Adam Hartmann-Kruckow
Steam-in-place (SIP) is a high-stakes sterilization process essential in many pharmaceutical environments – especially where disassembly is impractical. If you are navigating GxP compliance and wondering when SIP validation is required, how it works, and how to avoid common pitfalls, this guide breaks it down.
An easy overview of all the steps you need to perform reliable SIP validations.
Summary
Steam-in-place (SIP) validation ensures that equipment used in sterile pharmaceutical processes is properly sterilized using saturated steam without disassembly. It is required when microbial control is critical, and its validation must prove consistent delivery of sterilizing conditions. With regulatory pressure increasing and risk-based GMP frameworks evolving, SIP validation plays a growing role in operational efficiency, audit readiness, and digital compliance strategies.
What is steam-in-place (SIP) validation?
Steam-in-place (SIP) validation is a type of steam sterilization validation. It is a critical process in pharmaceutical and biotech operations that ensures sterile conditions within production systems – without disassembling equipment. It uses clean, saturated steam to sterilize internal surfaces of tanks, piping, valves, and other hard-to-reach areas after cleaning-in-place (CIP). This form of sterilization is essential for maintaining GxP compliance and protecting product integrity in highly regulated environments.
Unlike dry heat or autoclaving, SIP sterilizes non-portable equipment while it remains connected. The process must prove that steam reliably reaches and maintains at least 121 °C (250 °F) throughout the system for a specified duration. While a typical benchmark is 30 minutes, the actual hold time depends on achieving an F₀ value of at least 12 minutes – a measure of microbial lethality at 121 °C (250 °F).
Also read: Complete guide to steam-in-place validation in GxP environments
Who needs SIP validation – and when?
Steam-in-place validation is not necessary in every pharmaceutical or logistics operation. It is typically required in environments where microbial control and sterility are essential, and where in-place sterilization of equipment is more practical than disassembly.
You need SIP validation if you operate:
- Sterile or aseptic processing environments (e.g., biologics, vaccines, injectables)
- Sterile fill-finish lines where product-contact surfaces must be sterilized regularly
- Bioreactors and fermenters used in sensitive biotech production
- Multi-product systems requiring validated sterilization between campaigns
- Pharmaceutical-grade cleanrooms in logistics setups handling bulk drug substances or sterile transport tanks
SIP is likely not required if you:
- Manufacture non-sterile oral solid dosage forms (e.g., tablets, capsules)
- Use manual or autoclave-based sterilization with removable components
- Operate logistics sites without sterile handling or cleanroom areas
Determining whether SIP validation is needed depends on your facility design, regulatory classification, and the level of microbial control required by your processes.
How SIP validation works
At a high level, SIP validation ensures that clean steam is effectively delivered to all relevant surfaces and held at the required temperature for a validated period.
Some of the key parameters include:
- Uniform steam delivery to all product-contact surfaces
- Temperature monitoring to confirm target hold times (commonly 121 °C / 250 °F)
- Sensor placement in worst-case cold spots, often upstream of steam traps
Also read: Where to place data loggers during temperature mapping?
Regulatory and audit expectations for SIP validation
Depending on your operation, SIP validation must meet standards such as: GMP Annex 15 (qualification and validation guidance) ISO 17665 and EN 285 (moist heat sterilization requirements) FDA 21 CFR Part 211 (current good manufacturing practices for finished pharmaceuticals)
These define expectations for lethality values, equipment design, documentation, and periodic revalidation.
How to perform SIP validation
Steam-in-place validation typically follows the V-model of equipment qualification: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each phase builds on the last and helps ensure your sterilization process is fully controlled, documented, and compliant.
Also read: Temperature mapping and validation in GxP environments
The process begins by defining clear user requirements: Which systems must be sterilized, under what conditions, and to what standard. Once determined, the design phase assesses whether your setup – piping, traps, valves, sensors – supports consistent steam delivery and drainage.
1. Define user and functional requirements
This involves documenting the intended use of the equipment, identifying all product-contact surfaces, and setting clear sterilization goals based on the process risk and product profile.
2. Design Qualification (DQ)
Confirm that the SIP system design supports effective steam distribution and drainage Evaluate piping layout, steam trap placement, sensor access, and drain angles
3. Installation Qualification (IQ)
- Verify correct installation of valves, pressure gauges, traps, and sensors
- Ensure calibration of measurement instruments
4. Operational Qualification (OQ)
Perform empty chamber cycles to confirm control system performance Confirm that sterilization parameters (temperature, pressure) are achieved and held
5. Performance Qualification (PQ)
During PQ, validation runs are conducted under normal operating conditions, often with full product or simulated loads. Biological indicators are typically placed in cold spots to confirm microbial lethality, and temperature profiles are analyzed for uniformity and hold time compliance.
Also read:
- IQ, OQ, PQ in pharmaceuticals: Complete guide to equipment qualification
- IQ, OQ, and PQ services for GMP and GDP
Key setup considerations:
- Place sensors 300 – 450 mm upstream of steam traps
- Use multiple data loggers to capture all product-contact surfaces
- Simulate production conditions (with hoses, valves in normal positions)
- Validate F₀ values, dryness, and presence of non-condensables
Documentation should include test protocols, calibration certificates, deviation logs, and final qualification reports – all tied into your validation master plan.

Download a SIP validation checklist
Get a step-by-step checklist to help you conduct steam-in-place (SIP) validation reliably and aligned with regulatory expectations from GMP Annex 15, ISO 17665, and FDA 21 CFR Part 211.
Key decision triggers for SIP validation
SIP validation is not a blanket requirement for all equipment. It becomes necessary when specific process conditions, regulatory expectations, or equipment constraints demand in-place sterilization. This is particularly common in aseptic manufacturing and biologics production, where microbial control must be tightly managed.
Consider SIP validation if the equipment:
- Is part of an aseptic or sterile manufacturing process (e.g., injectables, vaccines)
- Requires sterilization without disassembly, such as reactors or piping systems
- Cannot be effectively sterilized using autoclaves or dry heat methods
- Is used in multi-product lines, requiring validated between-batch sterilization
- Falls under regulatory expectations such as those defined in GMP Annex 15 or by the FDA for critical utilities
Validation or re-validation is often triggered by:
- Installation of new systems or process lines
- Modifications to piping layouts, steam traps, or sterilization parameters
- Audit observations or internal findings indicating process gaps
Audit and regulatory impacts
From a regulatory standpoint, SIP is not optional when sterility is part of the product specification. Health authorities such as the FDA, EMA, and PIC/S expect manufacturers to validate that SIP systems deliver consistent, effective sterilization under real-world conditions.
This includes proving that:
- The sterilization temperature (typically 121 °C / 250 °F) is maintained throughout the system for the validated hold time
- Sensors are correctly placed in cold spots to capture worst-case data
- F₀ values are calculated to confirm microbial lethality
- Steam quality meets critical parameters: dryness fraction ≥90%, no non-condensables, and water-for-injection-grade purity
Tip! Also check out our SIP validation glossary right here.
All of this must be documented in your validation master plan and standard operating procedures. If your process fails to meet these requirements, consequences may include product rejection, delayed releases, or serious regulatory observations.
Where SIP fits in your compliance strategy
In facilities where sterilization is required, SIP sits at the intersection of quality assurance, operations, and regulatory compliance. It supports risk-based validation frameworks like GMP Annex 15 and ICH Q9 and ties directly into audit readiness.
Beyond the sterilization event itself, SIP connects to broader compliance efforts:
- It reduces contamination risk across multi-product systems
- It supports process consistency between campaigns
- It feeds audit trails with digital records of validated cycles and deviations
SIP is often coordinated with cleaning-in-place (CIP), temperature mapping, sensor calibration, and quality event logging as part of a holistic validation strategy.
Failure scenarios and pitfalls of SIP validation
Even well-designed SIP systems can fail validation.
Common causes include:
- Cold spots due to poor sensor placement or trapped condensate
- Inadequate steam distribution from blocked or undersized lines
- Non-condensable gases interfering with heat transfer
- Faulty steam traps causing premature condensate removal or accumulation
- Cycle interruptions (power loss, valve issues) disrupting hold time
- Lack of documentation or missed calibration windows
These risks can lead to non-sterile equipment, failed validation, and costly downtime. Prevent them with robust protocols, digital validation tools, and ongoing monitoring.
KPIs to evaluate SIP success
Like any GxP process, SIP validation should be continuously monitored and improved. The following performance indicators help teams assess whether their SIP system is operating reliably and compliantly:
- Hold time compliance rate: How often sterilization cycles meet the defined time and temperature thresholds
- Deviation count and resolution time: Track issues and how quickly they are resolved
- Audit readiness score: Assess completeness of documentation and traceability
- Revalidation frequency: Monitor how often changes or failures force a new validation
- Steam quality metrics: Confirm that steam consistently meets required dryness and purity standards
- Corrective actions: Log how often SIP-related failures result in CAPAs or process updates
Use this guide as a framework to build or assess your SIP validation strategy – and ensure your sterilization process does not put your GxP compliance at risk.
Step-by-step SIP validation checklist
Download a free step-by-step checklist to help you plan, perform, and document SIP validation in line with GMP Annex 15, ISO 17665, and FDA 21 CFR Part 211.

Steam-in-place validation services
Eupry provides GMP SIP validation services for pharmaceutical and biotech manufacturers.
- Fast project delivery
- GMP-specialized validation engineers
- Cost-effective services
Plus, everything else you need for thermal compliance - from autoclave validation to ongoing monitoring and calibration. One vendor, all you need for GMP compliance.
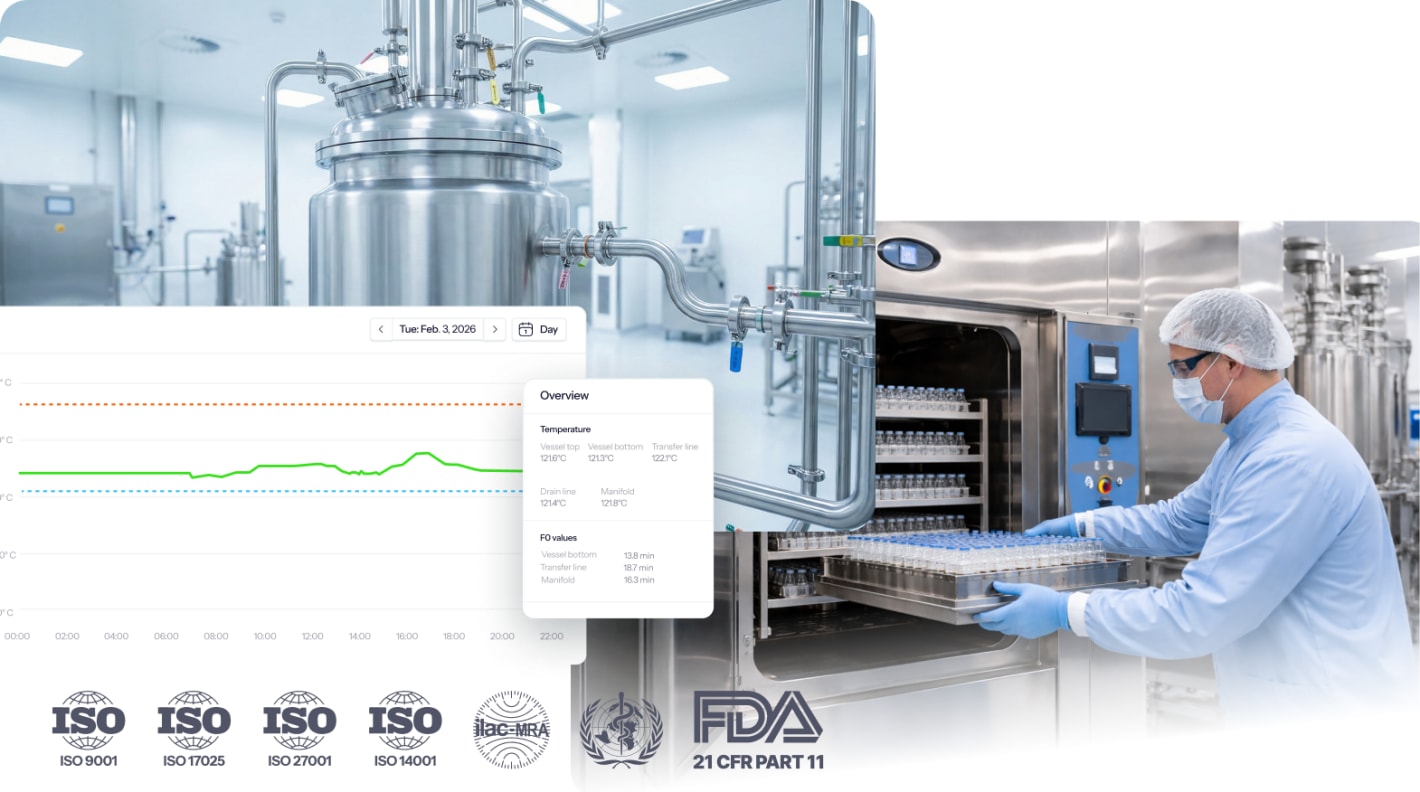
Get an instant overview of how the SIP validations services work.
Steam-in-place validation glossary
Need to make sense of steam-in-place (SIP) terminology fast? Quickly understand key terms in steam-in-place (SIP) validation for GxP environments. From F₀ values to steam traps, this glossary breaks down key terms and acronyms used in steam-in-place validation – especially for GxP environments.
Use the glossary as a quick reference as you explore SIP systems, design changes, audit prep, or qualification planning.
A
Autoclave: A pressure chamber used to sterilize equipment via saturated steam. Unlike SIP, autoclaving requires the equipment to be disassembled.
Annex 15: Part of the EU GMP guidelines that covers qualification and validation, including SIP requirements.
B
Biological indicator (BI): A test system containing viable microorganisms used to validate sterilization cycles by confirming microbial lethality.
C
Commissioning, Qualification, and Validation (CQV): A structured approach to bringing equipment and systems into a validated state. SIP validation often forms part of the qualification phases in a CQV framework. Learn more about CQV in pharma
Clean-in-place (CIP): The process of cleaning internal surfaces of equipment with chemical solutions before SIP sterilization. Cold spot: The hardest-to-sterilize location in a system. Sensors or BIs are placed here to confirm the effectiveness of the SIP cycle.
D
Design Qualification (DQ): Verification that the design of equipment or systems meets process and regulatory requirements.
Dryness fraction: A measure of steam quality. SIP requires ≥90% dryness to ensure effective heat transfer.
F
F₀ value: A calculated sterilization value that represents equivalent lethality at 121 °C. Used to validate SIP cycle performance.
G
GxP: An umbrella term for 'Good Practice' quality guidelines and regulations in the pharmaceutical and biotech industries. GxP includes GMP, GDP, and others, and ensures processes are traceable, documented, and compliant with regulatory expectations.
GMP (Good Manufacturing Practice): Regulatory framework that governs sterile processing. SIP validation must meet GMP requirements.
I
Installation Qualification (IQ): Confirms that SIP-related equipment has been installed correctly according to design and specifications.
N
Non-condensable gases (NCGs): Air or other gases that do not condense at sterilization temperature. These reduce steam effectiveness and must be minimized.
O
Operational Qualification (OQ): Testing the SIP system under empty-load conditions to verify temperature, pressure, and cycle control.
P
Performance Qualification (PQ): Verifies SIP performance in real operating conditions, including use of BIs and temperature mapping. PIC/S: Pharmaceutical Inspection Co-operation Scheme. Sets harmonized GMP standards across multiple regulatory agencies.
S
Steam trap: A device that removes condensate from the steam system without releasing steam. Critical for maintaining sterilization temperatures.
Steam quality: A measure of steam purity and effectiveness, based on dryness, pressure, and absence of contaminants.
Sterilization hold time: The period during which the SIP system must maintain sterilizing temperature to ensure microbial inactivation.
T
Temperature mapping: Recording temperatures at various points during SIP to confirm uniformity and identify cold spots. This is a core part of temperature mapping and validation strategy in GxP environments at various points during SIP to confirm uniformity and identify cold spots.
V
Validation Master Plan (VMP): A document outlining all validation activities, including SIP protocols, acceptance criteria, and responsibilities.