Pharmaceutical cold rooms

Adam Hartmann-Kruckow
Applications and GDP/GMP requirements
Understand the requirements and common applications for cold storage and how to qualify and monitor your storage facilities to meet GDP and GMP requirements for pharmaceutical cold storage.
Make sure your facility is GxP compliant and audit-ready with this step by step checklist.
What is pharmaceutical cold storage?
Pharmaceutical cold storage refers to temperature-controlled rooms and warehouses designed to keep medicinal products at validated conditions to safeguard the quality of these temperature-sensitive products.
Unlike domestic storage, these facilities must undergo formal qualification and continuous monitoring. Cold storage may include walk-in cold rooms, large warehouses, and distribution hubs.
Also read: The ultimate temperature mapping guide
Why does pharmaceutical cold storage matter for GDP and GMP compliance?
Cold storage protects patients and businesses by ensuring medicines remain effective. Non-compliance can lead to product loss, costly investigations, and regulatory findings. EU GDP and GMP Annex 15 emphasize that companies must validate storage areas and maintain monitoring systems to ensure integrity.
Common risks in cold storage include temperature excursions during door openings, poor air circulation creating hot and cold spots, and delayed alarm response. By addressing these, QA teams reduce deviations and prove control during inspections.
Also read: Monitoring pharmaceutical refrigerators: Logging, alarms, and audit-ready compliance
How are pharmaceutical cold rooms validated with IQ, OQ, PQ, and mapping?
Regulators expect that cold storage operates within validated ranges and that companies can prove this compliance. As such, cold rooms must be qualified before use and re-qualified at defined intervals through, among other processes, temperature mapping studies. This ensures the environment can maintain conditions under realistic operating scenarios.
Also read: Pharma cold room qualification (IQ/OQ/PQ)
Installation qualification (IQ)
Verifies installation against drawings and specifications, and documents all utilities.
Operational qualification (OQ)
Tests alarms, power recovery, and empty-chamber stability.
Performance qualification (PQ)
Verifies stability with product loads, door openings, and recovery to setpoint.
Temperature mapping studies
Identifies hot and cold spots using calibrated data loggers, with seasonal studies performed where required. Monitoring locations are defined based on mapping results. Tools like this data logger calculator for mapping help estimate the number of loggers required.
Learn more: Temperature mapping of pharmaceutical cold rooms
Re-qualification
Triggered by layout changes, HVAC modifications, or at intervals defined by risk assessment.
Also read: Key guidelines for temperature mapping


Thermal compliance for pharma cold storage
Get a structured way to evaluate whether your pharmaceutical cold storage rooms comply with GDP, GMP, etc.
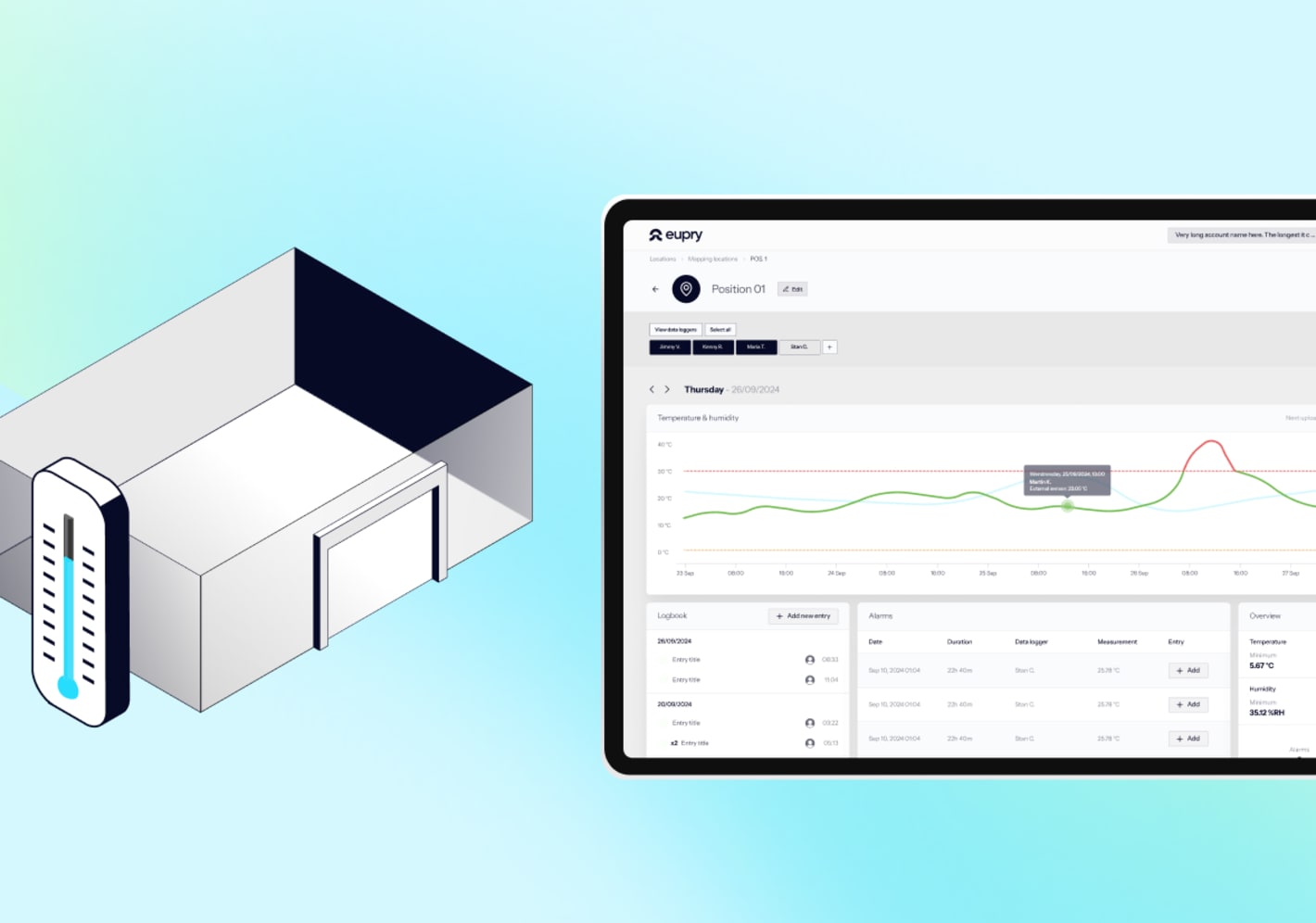
How should pharmaceutical cold rooms be monitored to stay audit-ready?
Monitoring is required by GDP to ensure that, once mapping is complete, conditions remain stable over time. Regulators expect permanent sensors to be positioned at identified hot and cold spots and for monitoring data to be secure and audit-ready.
Also read: How to master temperature monitoring in GxP
Continuous logging
Use calibrated data loggers or monitoring systems.
Alarm management
Define alert and action limits, test regularly, and document responses.
Calibration
Ensure all sensors are calibrated against national or international standards.
Data integrity controls
Validate monitoring systems against Annex 11 and 21 CFR Part 11, with audit trails enabled.
Audit readiness
Reports must be available to demonstrate compliance at any time.
What are the main applications of pharmaceutical cold storage in pharma and logistics?
Cold storage is used across the pharmaceutical lifecycle, from production to distribution. Typical applications include:
- Manufacturing sites: Holding finished products prior to release.
- Distribution centers: Consolidating medicines for national or regional supply.
- Hospitals and pharmacies: Storing vaccines and biologics close to patients.
- Logistics providers: Operating GDP-compliant warehouses and cross-docks for international shipments.
Each setting has different regulatory and operational challenges. For example, logistics providers often face seasonal mapping requirements, while hospitals focus on reliable alarms and deviation handling. Some companies are adopting continuous mapping to reduce requalification needs and streamline global quality programs.
Frequently asked questions about pharmaceutical cold rooms
Ensure your facility meets every GDP and GMP requirements
Get a temperature compliance checklist designed to secure GxP compliance of pharmaceutical cold storage facilities.

Cold room temperature mapping and monitoring solutions
Cold room monitoring and validation made simple. Keep your pharma facilities compliant and under control – without manual work.
- Instant SMS/e-mail alerts: Stop deviations before they cause issues.
- 3-click audit reports: GxP/ISO 17025/Part 11 documentation in clicks.
- Central control: Get one digital overview across sites and units.
