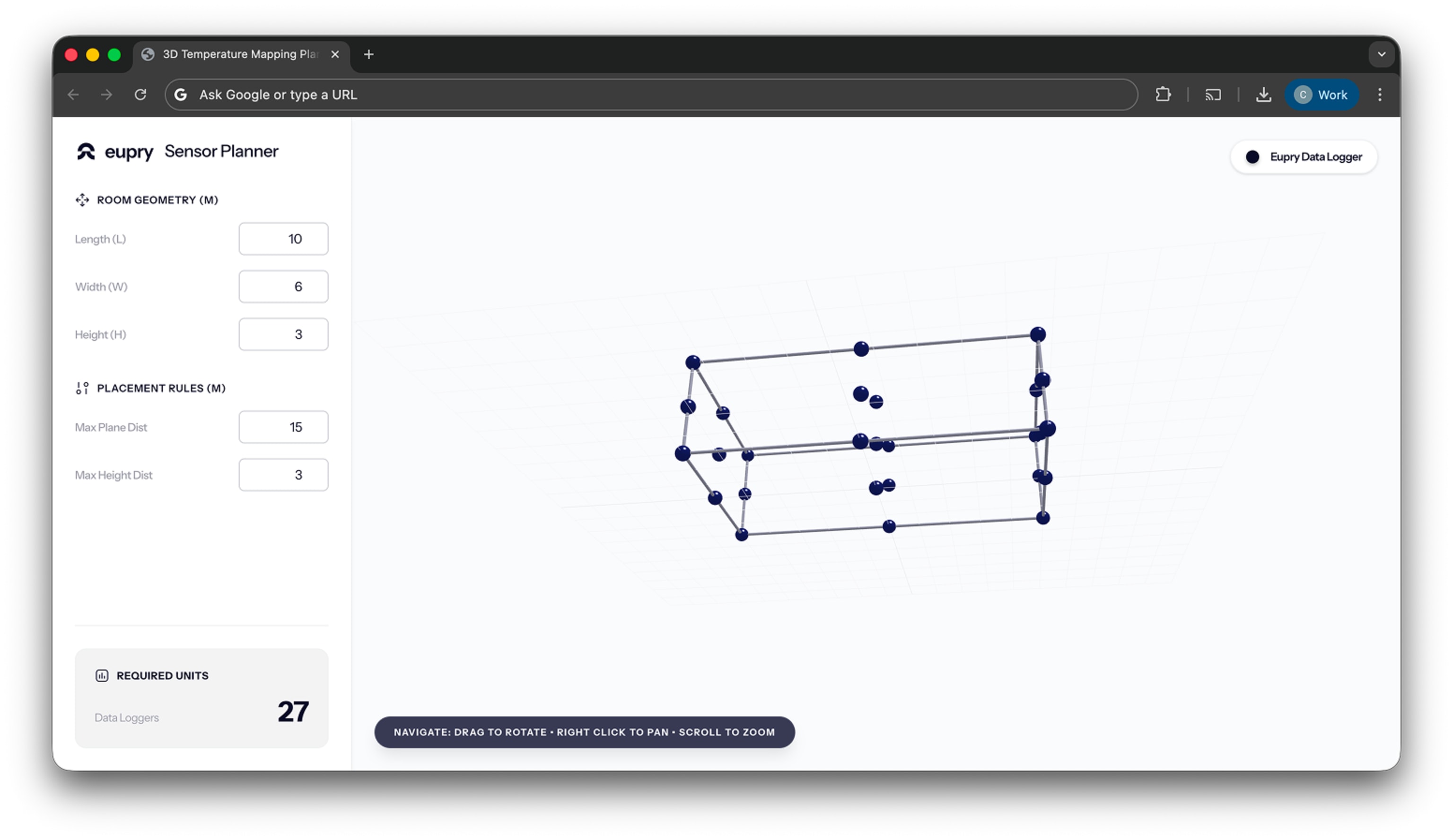
Featured article
Guidelines for continuous temperature mapping
The continuous temperature mapping framework offers an alternative to traditional validation. Learn how the method works, the key benefits, and the steps to make it part of your operations.

Articles

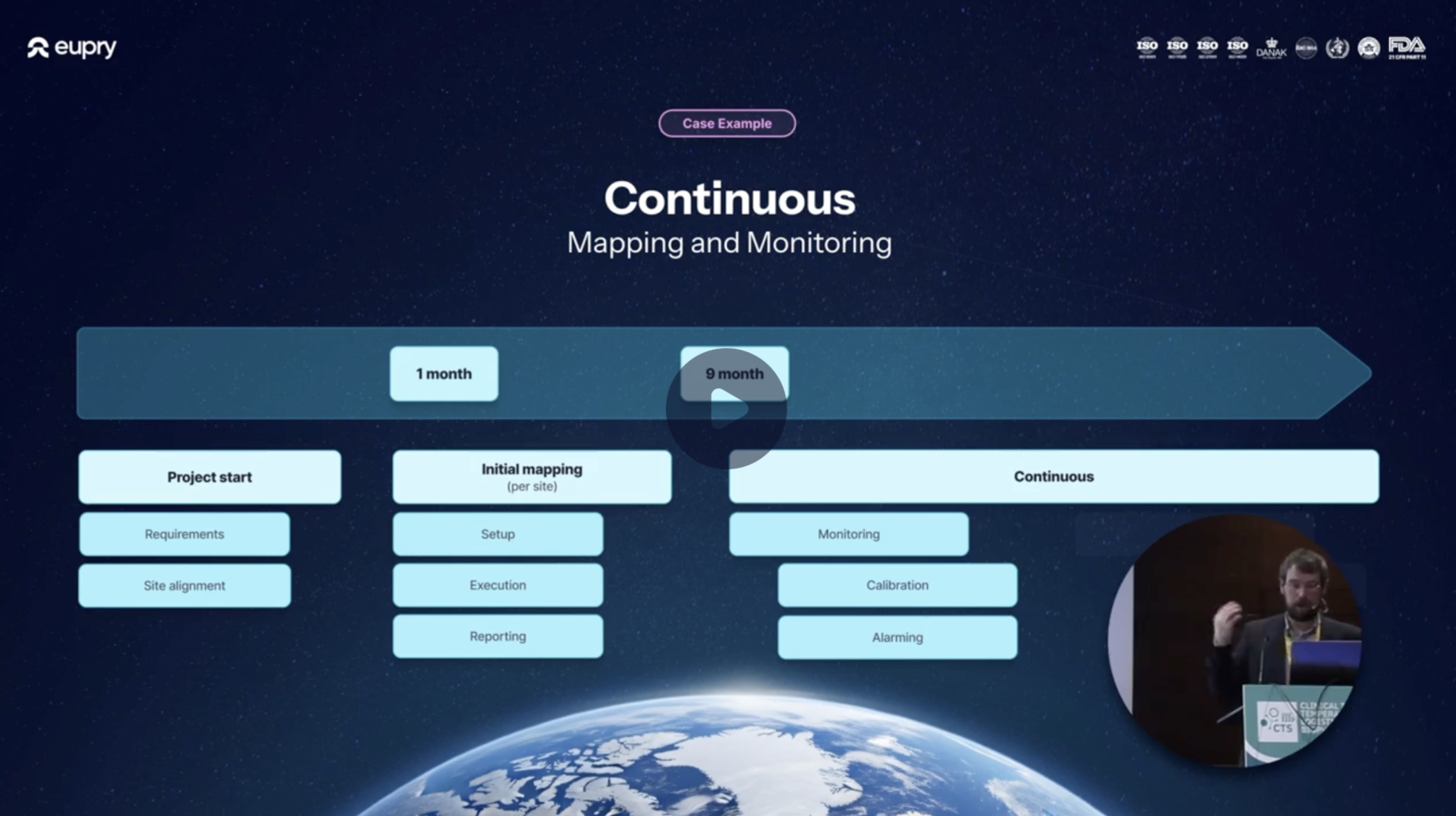
Continuous mapping (and monitoring) in practice

What QMSR means for labs using incubators

What is ISPE Validation 4.0?

Lyophilizer validation guide
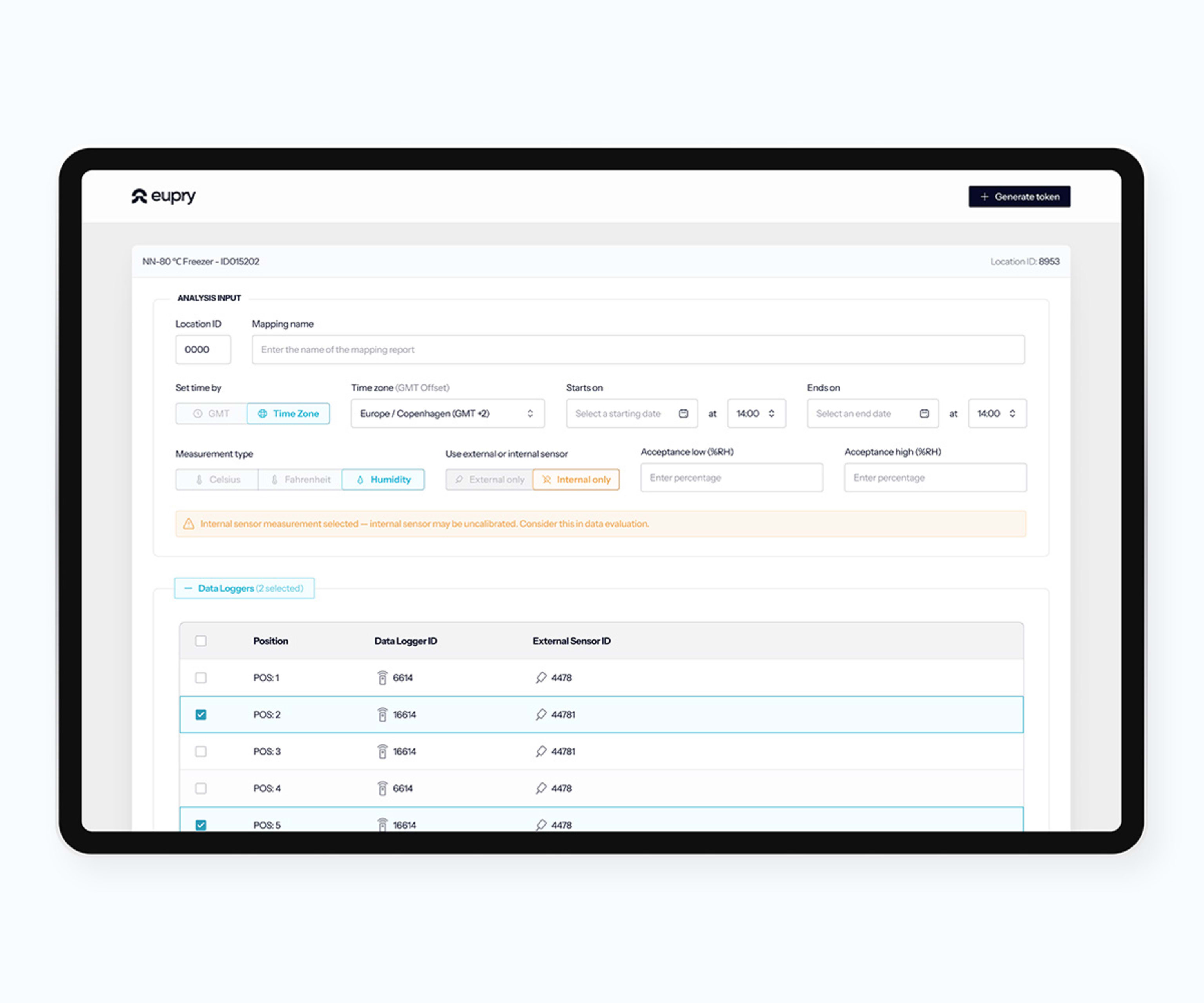
How to read temperature mapping data

Digital Calibration Certificates (DCC)

How to launch facilities before the second seasonal mapping

What to do when temperature mapping fails partially

What is pharmaceutical shipment monitoring?

The (possible) future of cold chain monitoring

Pharmaceutical cold chain monitoring

Autoclave validation guide

6 steps to standardize thermal compliance
How to harmonize temperature validation across multiple sites
How to meet GMP temperature requirements
How to choose an incubator monitoring system

How to handle thermal compliance for incubators
2025 Europe Tour
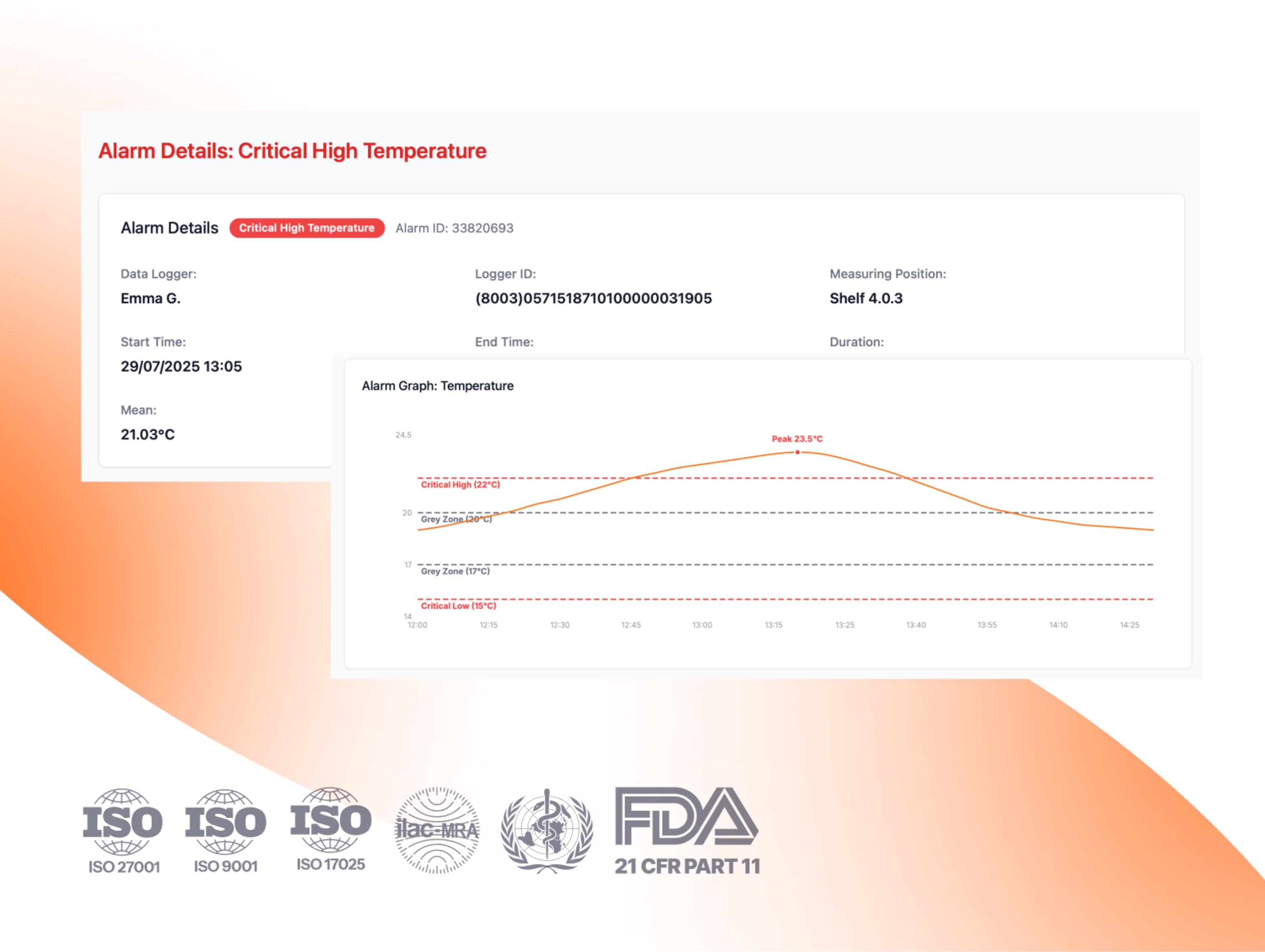
USP <1079.2> in action
ULT and freezer qualification and validation for pharma
More resources

Guides and checklist
Checklists, guides, templates, and more to help enhance your temperature compliance processes – from monitoring to mapping calibration.

Webinars
Watch live and on-demand webinars about everything from hands-on mapping guidelines to automating your compliance practices.

GxP glossary
Get a quick overview of the key terminology of temperature compliance and GxP – what it means, when it applies, and why it matters.