Certifications and accreditations
All your compliance boxes? Consider them ticked. Plus, we keep you ahead as requirements develop.
ISO 17025-accredited or traceable calibration
Simple, global calibration without downtime
No more worrying about calibration.
Our patented technology reshapes the way data logger calibrations are handled to greatly simplify the process.
- New calibrated sensors are sent when you need them to be replaced in seconds.
- Data is automatically merged and certificates are stored in the software.
- No extra loggers, no downtime, and no fragmented data.
Choose between ISO 17025 accredited or traceable 3-point calibration.
The calibration is included in the service with no extra costs.
Particularly convenient is the automated calibration process where external calibrated sensors are replaced with new calibrated ones automatically.
Eric Clausen Distribution Manager at Freja

ISO 9001-certified quality
With Eupry, you are choosing a partner who values quality as much as you do.
Our ISO 9001 certification underlines this commitment to quality management and customer satisfaction. You will experience processes and services that meet the highest quality benchmarks, essential for industries like logistics, pharma, and biotechnology, ensuring efficiency, reliability, and trust.

ISO 27001‑certified data security
Eupry is ISO 27001 certified, which means our system and processes are built to keep your data safe and compliant at all times. This certification demonstrates our commitment to confidentiality, integrity, and availability, giving you peace of mind that your compliance data is protected according to the highest international standards.
Data security is handled according to both ISO 27001 and Eudralex Vol. 4 Annex 11 Framework standards. All data is encrypted using AES and stored with continual backup procedures for as long as you need.
- Secure, encrypted storage for all your compliance data, fully aligned with ISO 27001 and Eudralex Vol. 4 Annex 11 standards
- AES encryption and continuous backups protect your temperature records, calibration certificates, and audit logs

ISO 14001-certified environmental management
Sustainable compliance you can count on
For GxP organizations focused on ESG reporting and sustainable supply chains, partnering with ISO 14001-certified vendors supports your own environmental commitments. Our ISO 14001 certification means environmental responsibility is built into how we operate and covers everything from products and services to waste reduction and resource efficiency.
Working with ISO 14001-certified partners helps you:
- Show environmental due diligence in your vendor selection
- Support your organization's sustainability goals
- Reduce complexity with a vendor who meets international environmental standards

FDA 21 CFR Part 11 compliance made easy
The system has a specialized 21 CFR Part 11 module and makes it simple to comply with the regulation.
- Fully validated system
- Digitally sign changes
- Export audit logs
And much more.

High compliance rating by Novo Nordisk
Eupry Aps provided (…) documentation to conclude that procedures and efficient controls to ensure compliance are in place and well-functioning as intended in all key areas/processes.
ISO Audit report by Novo Nordisk, just 2023

IT-validated compliance software your IT department will love
The monitoring software is fully validated – a critical factor for regulated industries like pharma and biotech. The validation ensures that the software meets industry standards and compliance requirements, reducing the risk of costly errors or data integrity issues.
Safe storage of personal data
Personal data is handled according to CCPA and GDPR standards, providing an extra layer of assurance for your data's security. In today's data-driven world, regulated industries cannot afford to take risks when it comes to personal data, and the service is designed to adhere to the highest standards supporting your compliance.

IQ OQ Qualification protocol included
The included Qualification Protocol helps you achieve GxP compliance with a custom-designed process for temperature monitoring.
This ensures:
- Proper installation of the monitoring system
- Correct configuration of all settings
- Effective procedures for alarm and deviation management

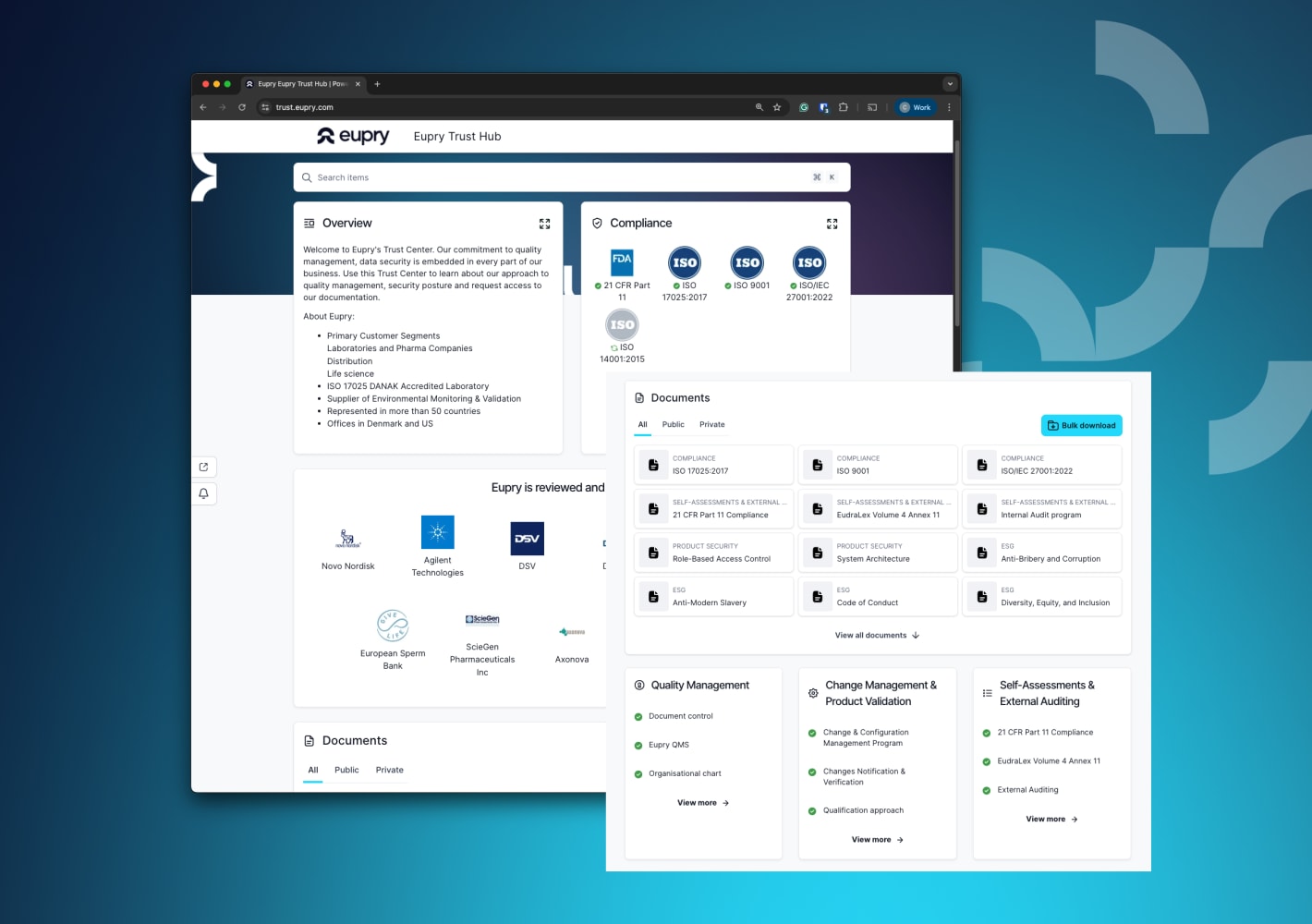
Visit the Eupry Trust Hub
Get an easy overview and access all the information you need to assess Eupry’s compliance documentation and processes.
Find, for instance:
- Certificates and accreditations, such as ISO 17025
- SOPs, policies, and company declarations
- Answers to typical audit questions and vendor questionnaires
And much more.
Automate thermal compliance with Eupry
Spend less time and gain full control of your temperature compliance with Eupry’s automated and connected solution – integrating monitoring, mapping, and calibration into one compliant package.
