Vaccine temperature data logger
Reduce risk, spend less time, and gain full control with reliable temperature data loggers designed for vaccines and other temperature-sensitive pharmaceutical products.
- Get instant e-mail/SMS alerts
- Print audit reports in 3 clicks
- Monitor from anywhere, on any screen
- Handle calibration in minutes directly on site

Get instant access to all the technical specifications, data loggers options, and more.

Trusted by +500 pharma and healthcare logistics companies including:
Tailored for pharma storage
With up to 50% of vaccine inventory discarded globally due to storage issues, reliable monitoring is vital.
The wireess data loggers and ISO-accredited solution is built for vaccines and other types of highly regulated pharma products and provide you with dependable, real-time temperature and humidity monitoring.
Don't take our word for it
"We have saved around 50-70% of the time we spend on temperature monitoring."
Anders Rasmussen Senior Logistic Manager, Dechra Pharmaceuticals PLC
Client Testimonials
How do vaccine temperature data loggers work?
- The vaccine data loggers work by constantly measuring temperature and humidity and automatically sending the data to the included monitoring platform via Wi-Fi.
- The advantage of having a wireless data logger compared to the USB logger is that the wireless can transmit data automatically without the need of manual work.
- The wireless data loggers send alarms immediately if excursions occur. This means that you can take the correct action, prevent costly vaccine spoilage, and ensure continuous compliance.

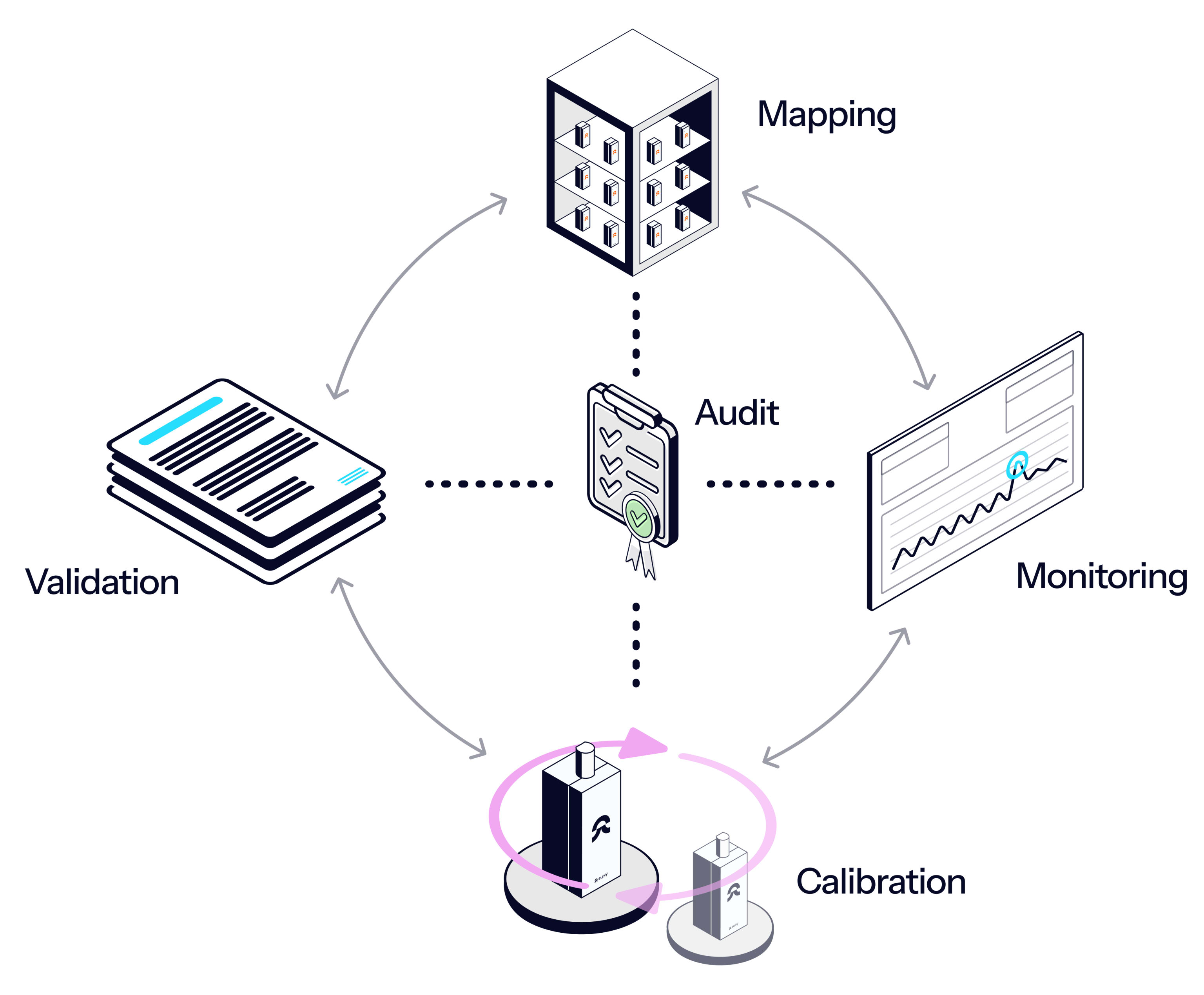
All you need for thermal compliance
Eupry is more than monitoring. It is a digital system that gives you full control over all your temperature compliance efforts and collects all data in a single view – from vaccine monitoring to calibration and mapping.
Less time sifting through the mess
3-click audit reporting, easy access to all the data you need, and digital storage of ALL your temperature data – from calibration certificates to temperature records and deviations. With everything accessible, it is simple to find the information that matters most.
Enjoyable audits
From ISO 17025 to ISO 27001 and ISO 9001; rest easy, you are fully compliant - and you can export full audit reports in just 3 clicks.
Minimized mistakes
Removing mundane tasks means removing manual errors. We believe in compliance confidence.
Full overview
Complete overview of every step of the monitoring process. Control the process to control the quality.
Ease of use
Why has temperature compliance become so complex? The solution is easy to install, learn, use, and pitch internally.

Download a free product catalog now
See all the technical details about the wireless vaccine data loggers and how the temperature compliance solution works.
Free and automated calibration without swapping data loggers
Our patented calibration technology offers notable time savings by automating the process and eliminating the need for replacing your data loggers.
The system monitors your loggers’ calibration status and when it’s time, we simply send you newly calibrated sensors to replace the old ones.
Forget spare loggers and their limitations, calibrate all your gear at once, and leave behind the stress of tracking calibration statuses. Of course, you can also get an overview of your logger’s status with a few clicks.
But you don’t have to. We’ve got your back.
Unlike traditional calibration that causes downtime and requires spare loggers, our on-the-wall process keeps your monitoring active while calibration happens

Automated monitoring from one place
= one platform, all your data.
Track compliance on any screen – from your desktop to your phone. The monitoring platform gives you a full overview across units and facilities.
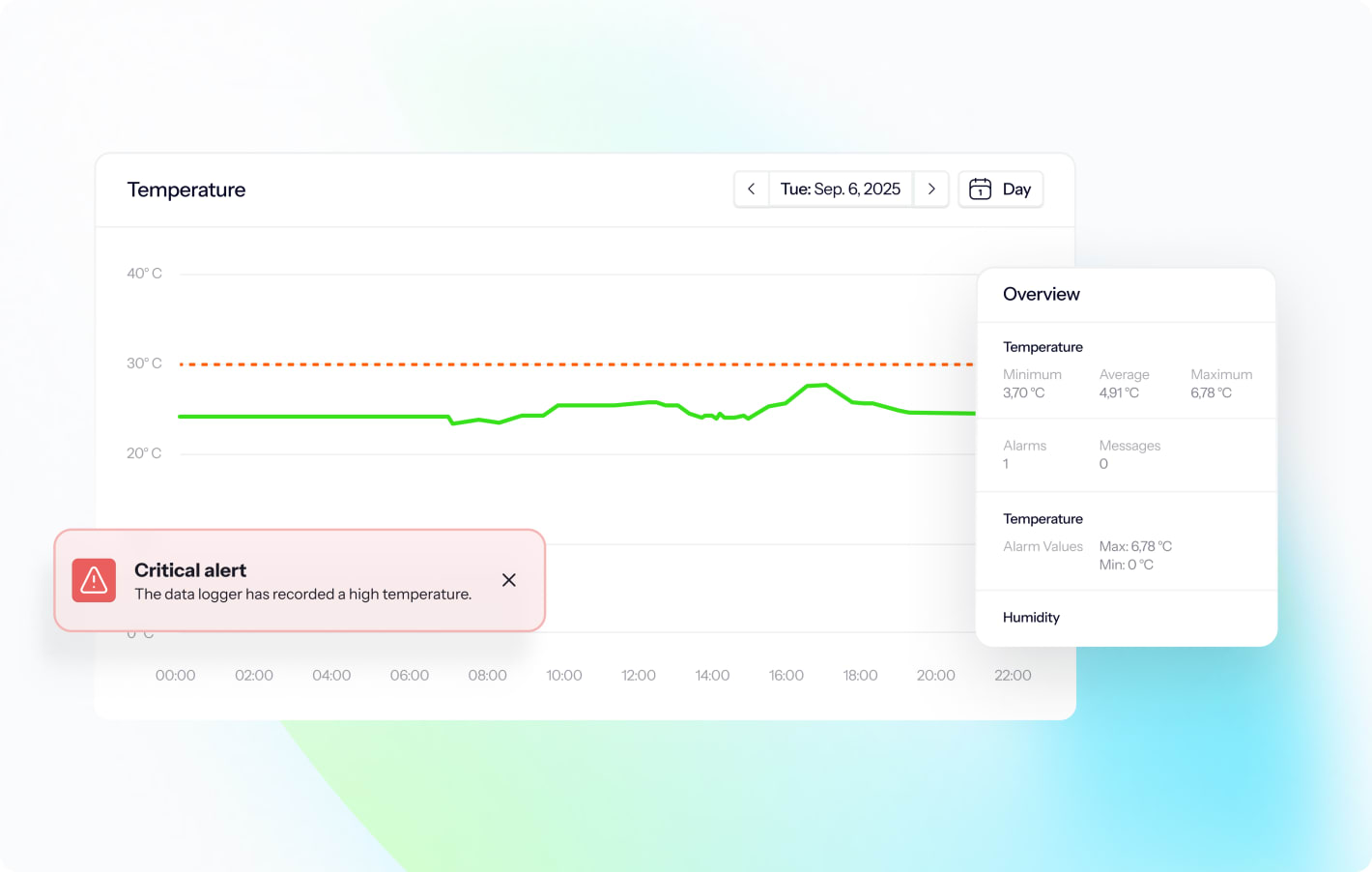
SMS/email alerts
Handle deviations fast and according to GxP requirements to protect your vaccines and other sensitive pharma products.
- Reduce noise: Separete grey zones from critical alerts and personalize who gets what alerts and when.
- Act faster: Get instant alarms on SMS and email.
- Log easier: Alarms are automatically logged in the deviation management tool to easily create NCRs.

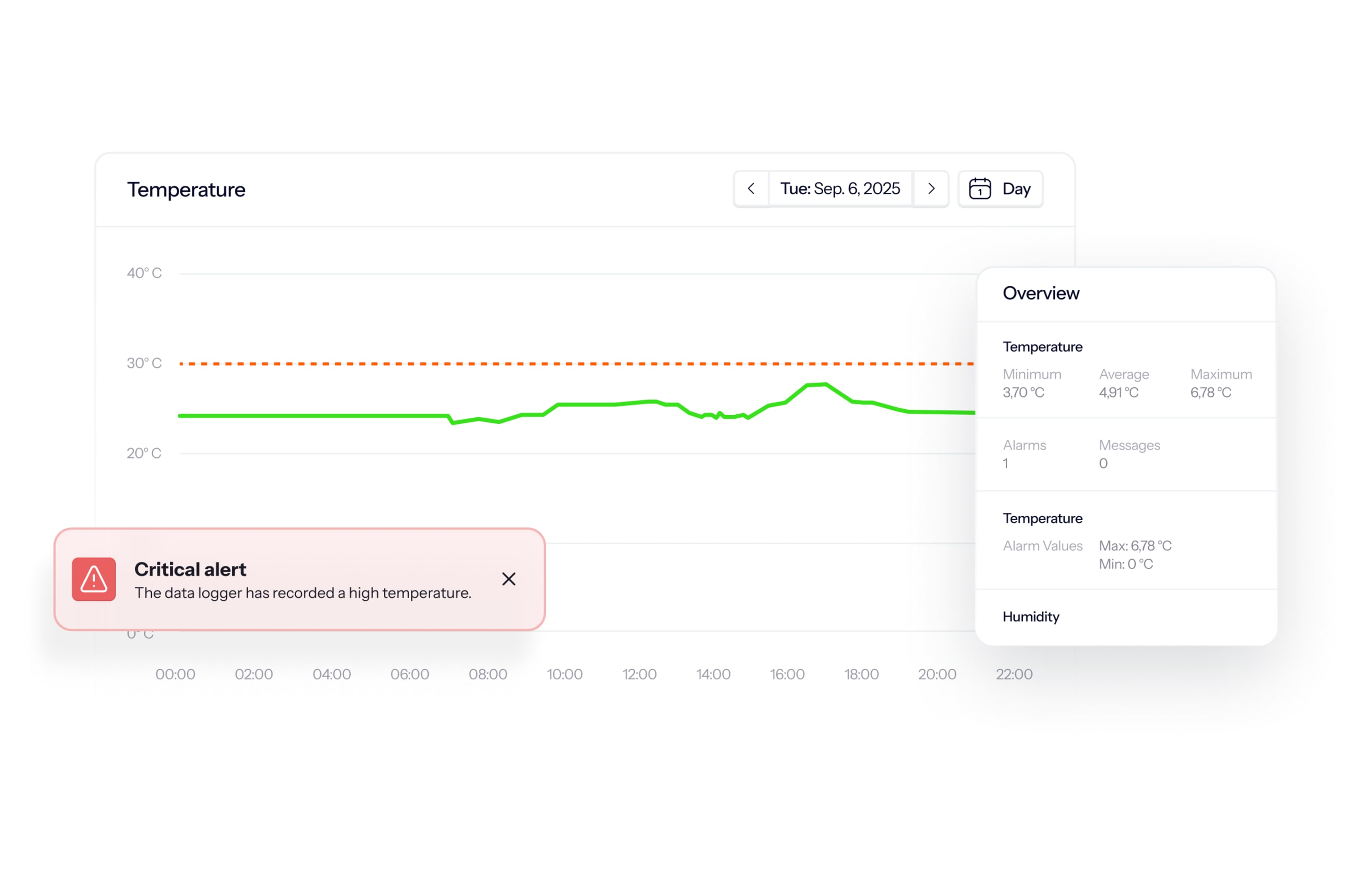
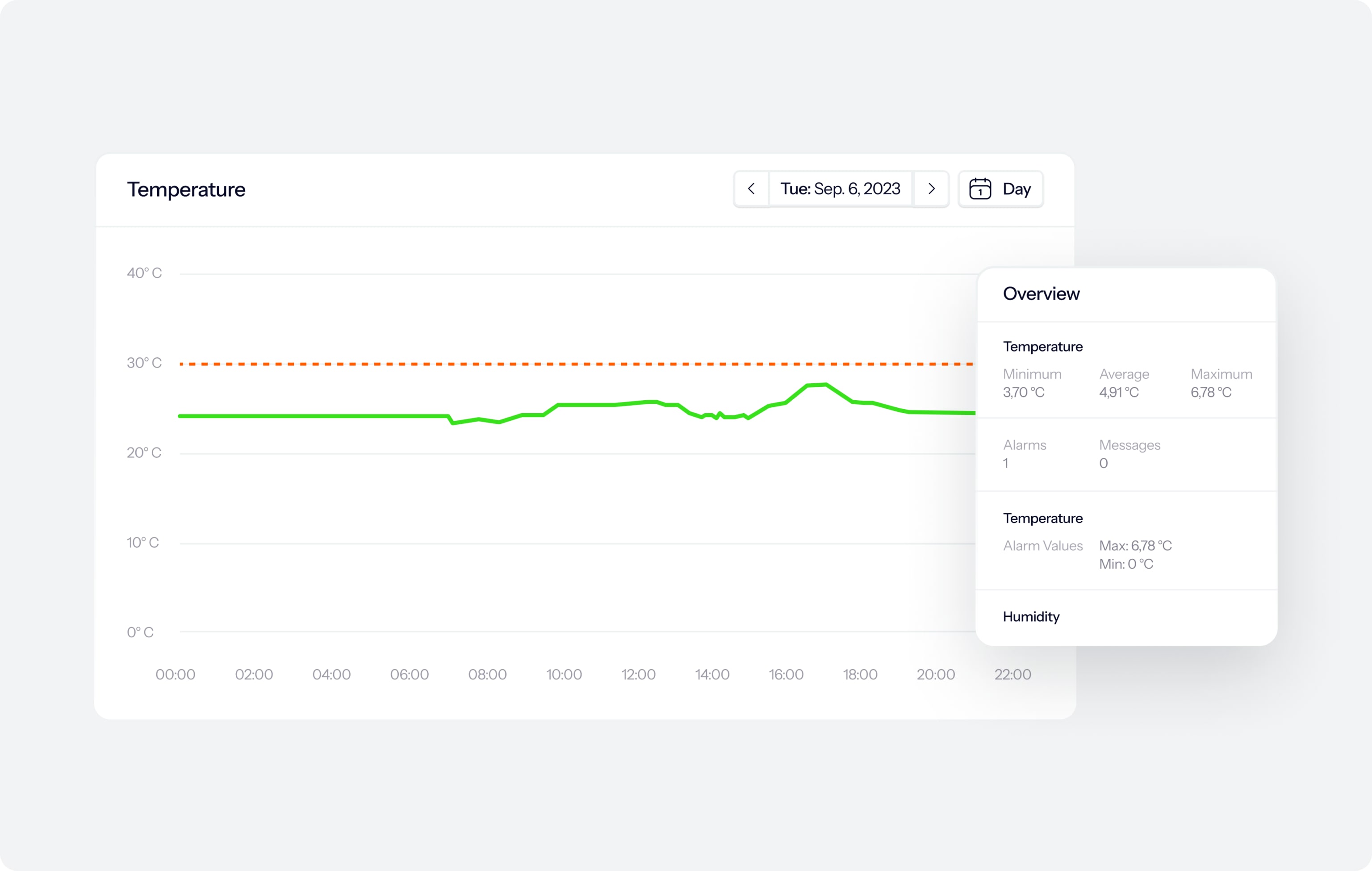
How the monitoring software works
Watch real-time and historic measurements in a user-friendly, IT-validated online environment. In the software, you can, for instance, find
High compliance rating by Novo Nordisk
Eupry Aps provided (…) documentation to conclude that procedures and efficient controls to ensure compliance are in place and well-functioning as intended in all key areas/processes (…)
ISO AUDIT REPORT by Novo Nordisk, June 2023

“A short way of describing our experience with the solution is simply that it is really easy to use. Both to get started and to work with in day-to-day operations.”
Dorte Egeberg & Allan Toft Jacobsen, Lab Specialist & COO at European Sperm Bank
Client Testimonials
Wireless vaccine data logger sensors
These are just examples - find all options in our free product catalog.

ALIIO Temperature Sensor
Operating range: -50°C to +50°C (-58°F to 122°F) Temperature resolution: 0.01°C (0.018°F)

ALIIO Humidity Sensor
Operating range: -50°C to +50°C (-58°F to 122°F) Humidity operating range: 0-99% RH

ALIIO Temperature Probe
Operating range: -90 °C to +50 °C (-130 °F to 122 °F) Temperature resolution: 0.08°C (0.14°F)

External Temperature Teflon Probe
Temperature operating range: -200 °C to +200 °C (-320.8 °F to 392 °F) Temperature resolution: 0.03 °C (0.054 °F)
The compliance components
All your compliance boxes? Consider them ticked. Plus, we keep you ahead as requirements develop.














