USP <1079.2> in action
How Eupry’s new alarm handling provides faster, safer excursion evaluation
Eupry’s new alarm handling system for temperature monitoring puts USP <1079.2> into practice with compliant thresholds, validated delays, and an audit-ready trail, as well as providing better incident handling functionalities.
Here is how it works.
Get all the technical specifications and see how the monitoring and alarm system works
Short version
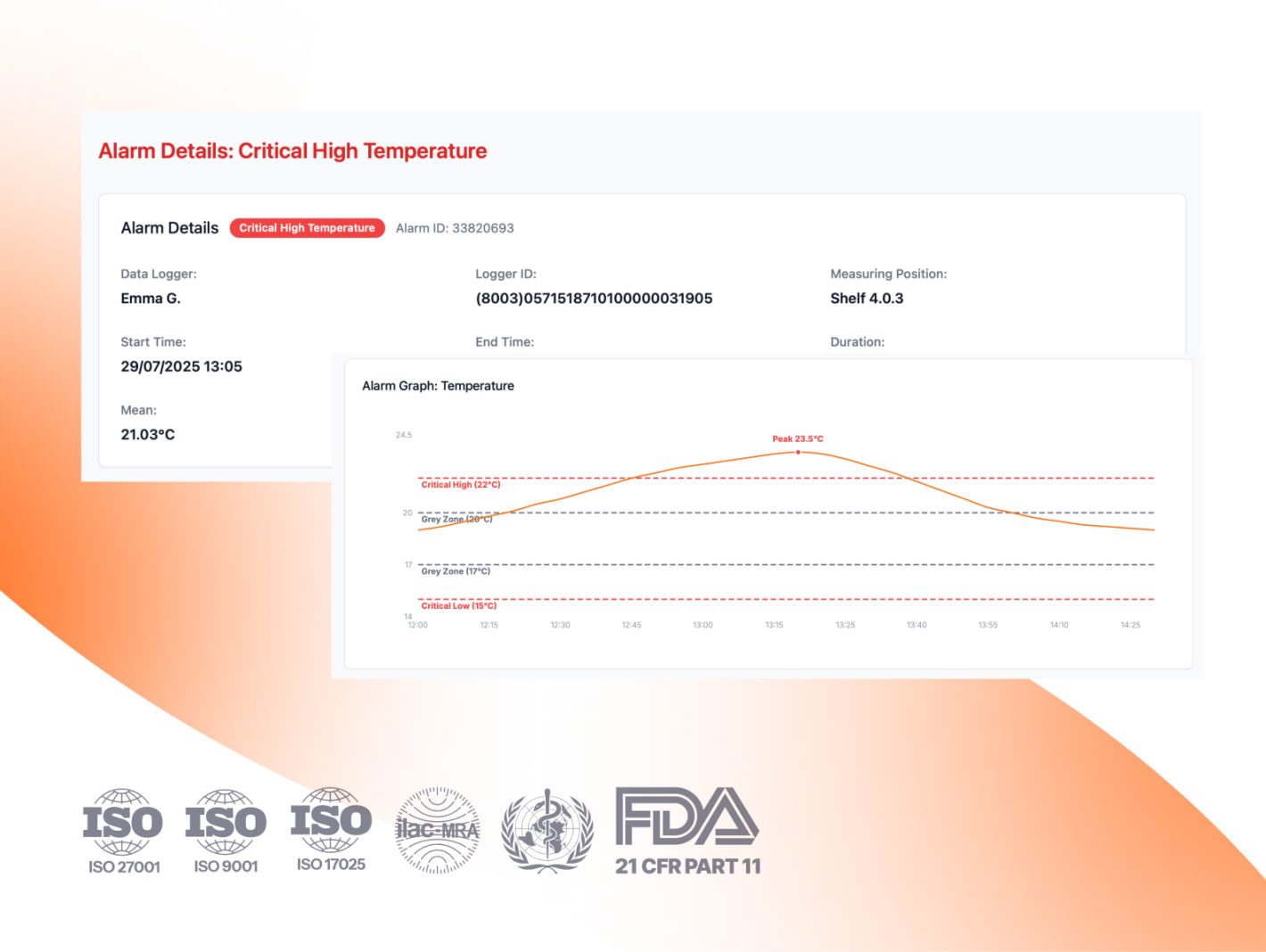
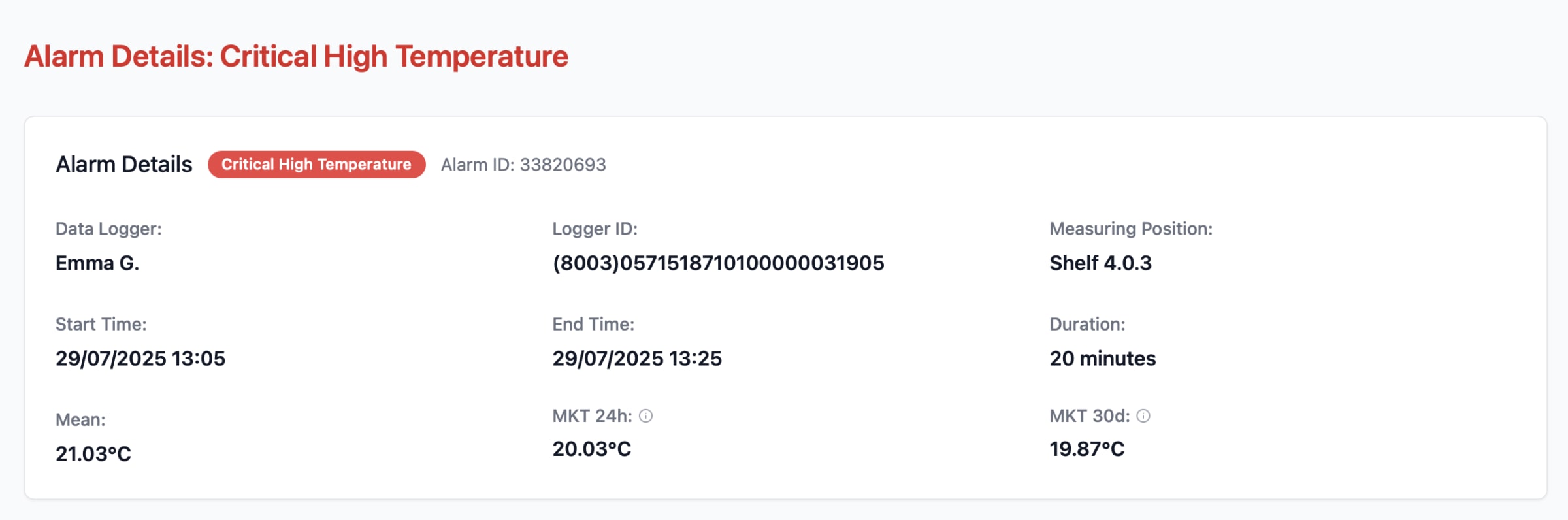
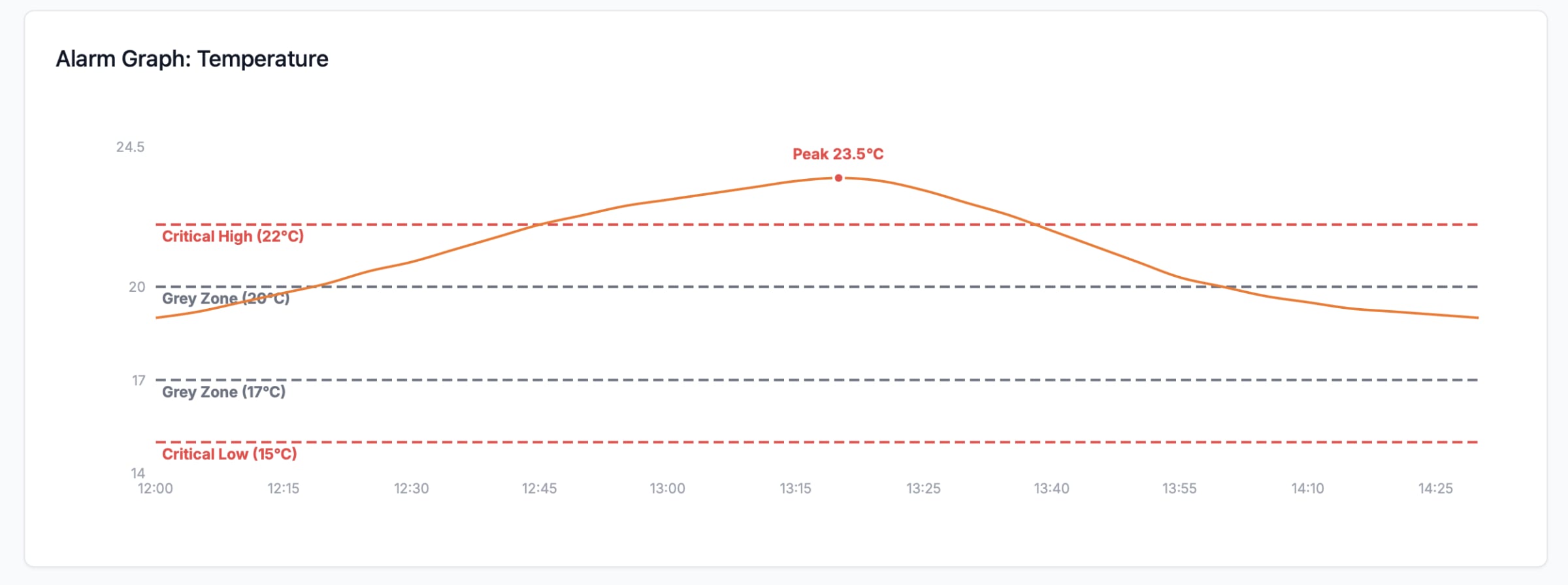
Eupry’s new Alarm Details page automates mean kinetic temperature (MKT) calculations using the 30-day (CRT) and 24-hour (CCT) windows recommended in USP <1079.2>. This removes the need for manual Excel analysis, giving QA teams faster, consistent, and audit-ready excursion evaluations.
Download a product catalog or learn more about how the monitoring system works here.
Pharmaceutical quality teams are under growing pressure to manage alarms in a way that satisfies regulators, avoids unnecessary workload, and supports confident release decisions.
USP <1079.2> has brought welcomed clarity on how to evaluate excursions using mean kinetic temperature (MKT), but it leaves QA teams with the practical challenge of translating those rules into day-to-day operations by performing statistical analysis on the data in, for example, Excel.
Eupry’s new alarm handling system closes this gap by making alarm management compliant, validated, and audit-ready – while reducing valuable QA time. Here is how it works.
Also see: 7 updates from USP <1079.2>: How to evaluate temperature excursions with MKT
Why does USP <1079.2> matter for alarms?
USP <1079.2> clarifies how pharmaceutical companies should evaluate temperature excursions using mean kinetic temperature (MKT). It emphasizes the need for scientifically justified evaluation windows and documented decision-making. However, it does not prescribe exact alarm thresholds or delay times. QA teams must still determine when to trigger alarms, how to apply mapping results, and how to document decisions in a way that will satisfy inspectors. This is why alarm management has become a compliance pressure point.
Also read: 7 updates from USP <1079.2>: How to evaluate temperature excursions with MKT
What is Eupry’s new alarm handling system?
Eupry’s new alarm handling system is a new module within our monitoring designed to make excursion handling both easier and faster and make it easier to translate USP <1079.2> expectations into daily practice.
Key features at a glance
In plain terms, it is:
- A validated alarm management framework: Built to meet GDP, GMP, Annex 11, and 21 CFR Part 11 requirements.
- Configurable thresholds and delays: Alarm limits come directly from mapping studies and USP <1079.2> MKT guidance, with rational delay and hysteresis settings to reduce false positives.
- Smarter alarm logic: Cuts down alarm fatigue by filtering non-actionable alarms while keeping full compliance evidence.
- Audit trail and periodic review: Every alarm, acknowledgement, and comment is recorded, traceable, and reviewable in line with regulatory expectations.
- Fully digital and harmonized across sites: One SOP, system, and audit-ready overview across all facilities and countries.
In short, it turns USP <1079.2> alarm expectations into a practical, validated, and audit-proof system for QA teams.
Release video
What problem does Eupry’s alarm handling system solve?
GxP companies often struggle with two extremes: receiving too many unactionable alarms or missing critical ones due to poor configuration. Both create compliance risk and drain resources.
Eupry’s new alarm handling system ties directly to USP <1079.2> expectations, reduces unnecessary noise, and generates audit-ready documentation.
How does the system turn USP <1079.2> into practice?
Eupry connects regulatory requirements with everyday QA workflows. The system helps QA managers and validation engineers move from theory to practice:
Set compliant thresholds
Alarm limits are derived from mapping results, controlled room temperature (CRT) and controlled cold temperature (CCT) definitions, and USP <1079.2> MKT evaluation windows.
Maintain digital audit trails
Every alarm, acknowledgement, and corrective action is automatically logged in line with Annex 11 and 21 CFR Part 11 requirements.
How does this help QA teams in practice?
Instead of drowning in alarms or relying on spreadsheets to justify release decisions, QA teams can use Eupry’s monitoring and alarm handling system to act faster and with more confidence.
The system reduces time spent on deviation investigations and lowers the risk of audit findings. By harmonizing alarm logic across all facilities, it also makes multi-country operations more consistent and predictable – a growing need as Annex 11 revisions and global GDP enforcement raise expectations.
What can you expect to get out of the system?
Implementing Eupry’s alarm handling system provides measurable value:
- Reduced noise: Fewer non-actionable alarms and less alarm fatigue for QA and operations staff.
- Audit confidence: Digital records, acknowledgements, a new initial cause analysis, and sign-offs create a defensible compliance history that aligns with Annex 11, Part 11, and WHO TRS 961.
- Faster investigations: Excursions are evaluated against USP <1079.2> windows in minutes, not hours, with decisions documented for inspectors.
- Standardized processes: Harmonized SOPs and one system for alarm management across facilities worldwide.
- Lower compliance risk: Clear, documented rationale for every alarm, reducing uncertainty during FDA, EMA, or MHRA inspections.


Download a free product catalog
Get instant access to an overview of how the monitoring system, alarm management system, etc. works in our free product catalog.
How can you simplify alarm management in your operation?
The alarm handling system is part of Eupry’s monitoring and full temperature compliance solution. If you are ready to cut false alarms, reduce audit risk, and make excursion evaluation faster, our team can show you how. Learn how the new alarm handling system works in practice and how it can help your QA team gain time and confidence.
