Remote temperature monitoring system for GMP and GDP
Reliable wireless temperature monitoring system built for GxP compliance.
- Real-time and remote monitoring on any screen
- All-in-one GxP-compliant solution
- Create audit reports in 3 clicks
Download a free catalog and get all the details and an easy overview of your options.
+1000 GxP customers worldwide benefiting from Eupry
Traditional temperature monitoring is manual and error-prone
No remote visibility
You can’t fix what you can’t see. Without real-time access to monitoring data, you are left reacting to issues too late – or not at all.
Manual work
Too much time is lost to transferring data and searching through folders. It is slow, error-prone, and frustrating for your team.
Audit stress
When regulatory inspectors arrive, missing records, scattered data, or inconsistent reports create unnecessary risk.
Disconnected systems
Monitoring records in one place, calibration somewhere else, and mapping in another system, with no central overview.
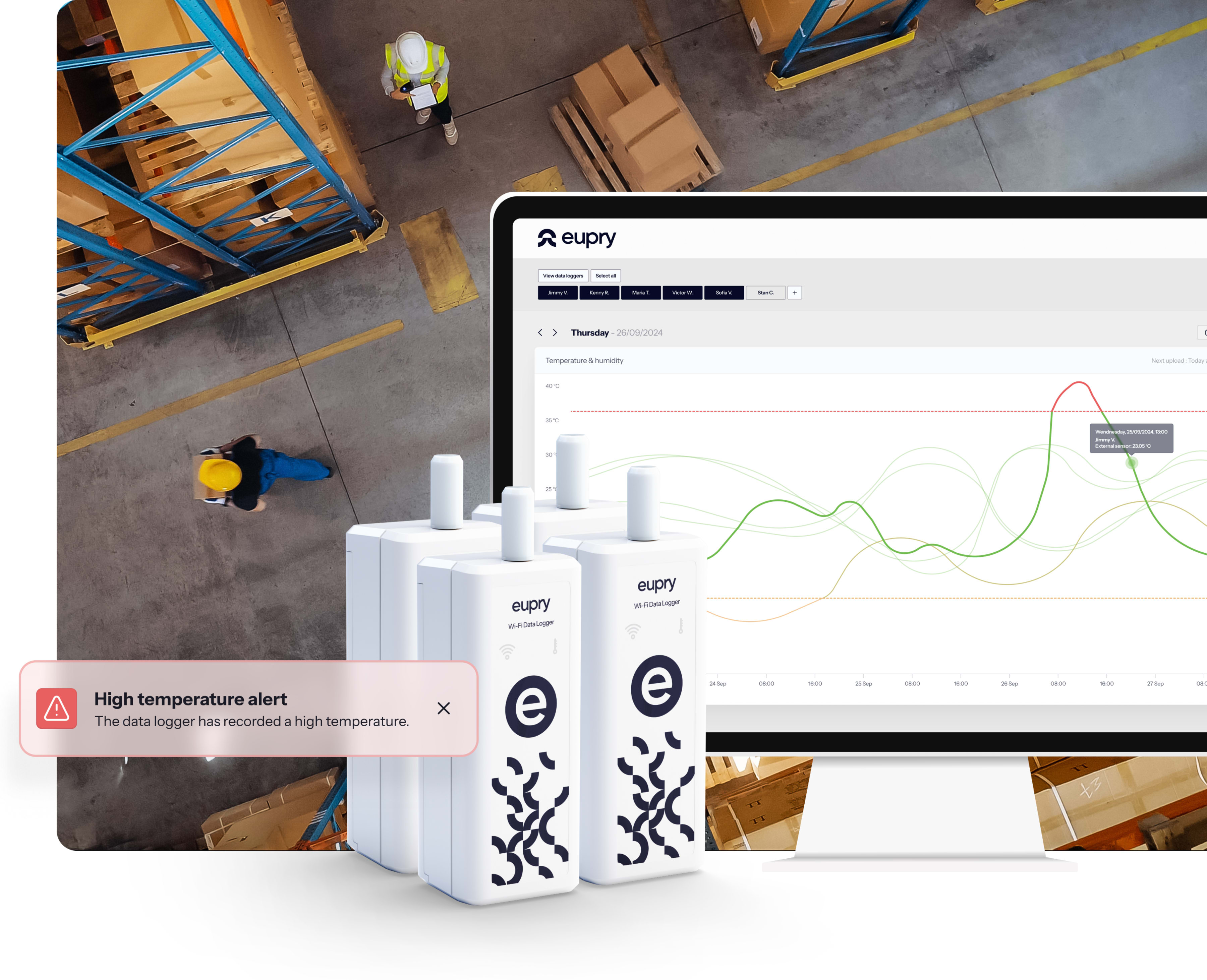
Live remote monitoring built for GxP – from anywhere
With Eupry, you get one GxP-compliant platform for monitoring, reporting, and real-time and remote control across all your sites.
Monitoring from anywhere
Get instant deviation alerts and monitor all your sites in real time on any screen - phone, tablet, and computer.
Fully automated workflow
No more manual tasks. Let the system do the work: Automate data logging, deviation handling, and full (audit) reporting.
Audit ready in 3 clicks
Export full reports with everything you need for a successful audit – from temperature data to deviation reports and certifications – in just a few clicks.
All-in-one platform
Gather monitoring, calibration, and validation in one digital platform. No more scattered systems or multiple vendors.

One platform, all your data - from anywhere
Data, certificates, reports, and audit trails are securely stored in a cloud-based platform, allowing for instant digital access to all documents - from anywhere and any screen (mobile, dektop, tablet).
Whenever you need them, wherever you are.

Wireless Wi-Fi data loggers built for GxP
The data loggers automatically send data through Wi-Fi to your (included) platform and let you monitor your environment – from fridge to warehouse – without manual data transfers and in real-time.
- Instant deviation alerts on SMS/e-mail
- No manual data transfers or alarm logging
- Validated backups ensure data integrity (even if Wi-Fi is out)

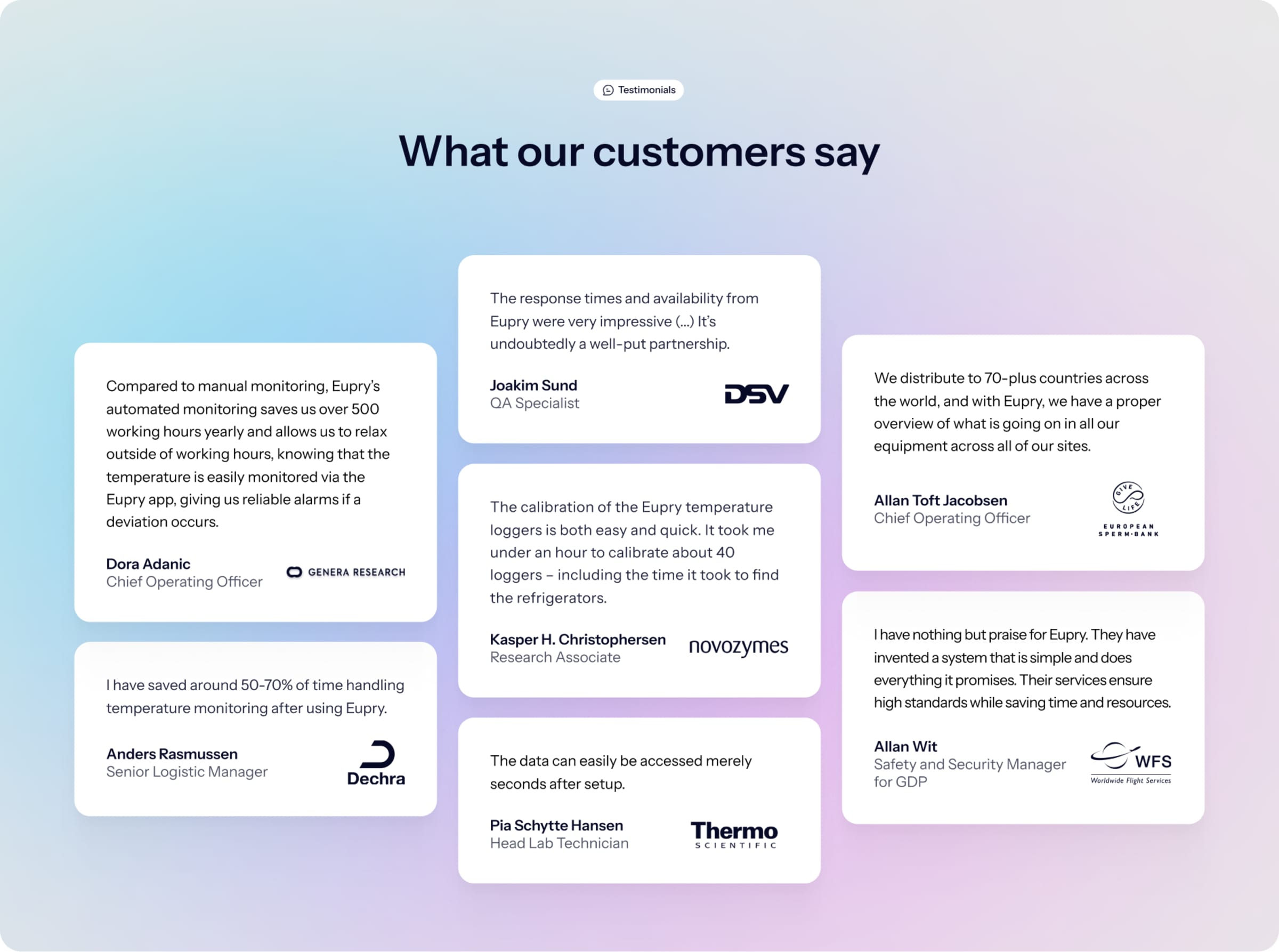
Save 500 working hours yearly with automated temperature monitoring
"Eupry is a time saver. In comparison with manual temperature monitoring, Eupry saves us more than 500 working hours yearly, allowing us to use that time for other important tasks."
Dora Adanic, Chief Operating Officer at Genera Research

Download your free product catalog
See how the remote monitoring solution works, get access to all technical specifications, and find all product options in our free catalog.
Remote monitoring for healthcare logistics
Eupry’s wireless temperature monitoring system is designed for the specific challenges of healthcare logistics. Ensuring the proper temperature throughout the supply chain is critical in order to protect sensitive goods and maintain compliance. The system is built for small and large spaces with remote, reliable data logging that can be accessed anywhere, anytime.
- Robust for large facilities: Wireless setup, Wi-Fi-based data loggers, and robust connectivity mean that the solution easily accommodates large spaces. Data loggers have unique names to make them easier to locate.
- Tailored support: Our compliance specialists can help you define everything from the optimal setup based on your specific floor plans to the diverse needs of different products.
- Remote access: No need to access individual devices, which can be pretty neat in a big storage space, and data can be accessed from anywhere at any time.
- GDP compliance: The system is fully aligned with industry standards such as the FDA’s enforcement of GDP, and compliance levels are flexible catering to various goods and helping to control costs.

Remote monitoring for pharma
Eupry’s solution is designed for GxP-compliant and audit-ready reporting with only a few clicks. Our dedicated compliance specialists keep up with every regulatory turn to keep the solution – and you – a step ahead.
- Simple audit processes: Digital tools to export audit-ready reports with a few clicks.
- Compliance in the forefront: ISO 17025-accredited calibration, ISO 9001 accreditation, ISO 27001 security, and a specialized FDA 21 CFR Part 11 module.
- Continuous data integrity: Reliable backup systems, secure storage, user access control, and much more.
- From lab settings to mass production and storage: Adapting to the needs of the entire pharmaceutical value chain – from R&D to GMP and GDP.

Remote monitoring for biotech
Be in full control of your temperature compliance at all times. Collect temperature and humidity monitoring, mapping, and calibration in one GxP-compliant solution – or handpick the components you need.
- Accuracy: High-precision sensors support good laboratory practice (GLP) with accredited calibration.
- Simplifying auditing: Robust deviation management and reporting tools.
- Scalability: The subscription model, easy installation, and flexible wireless setup accommodate the rapid growth of many biotech operations.
- Automated data recording: Ensuring data integrity and real-time alerts.

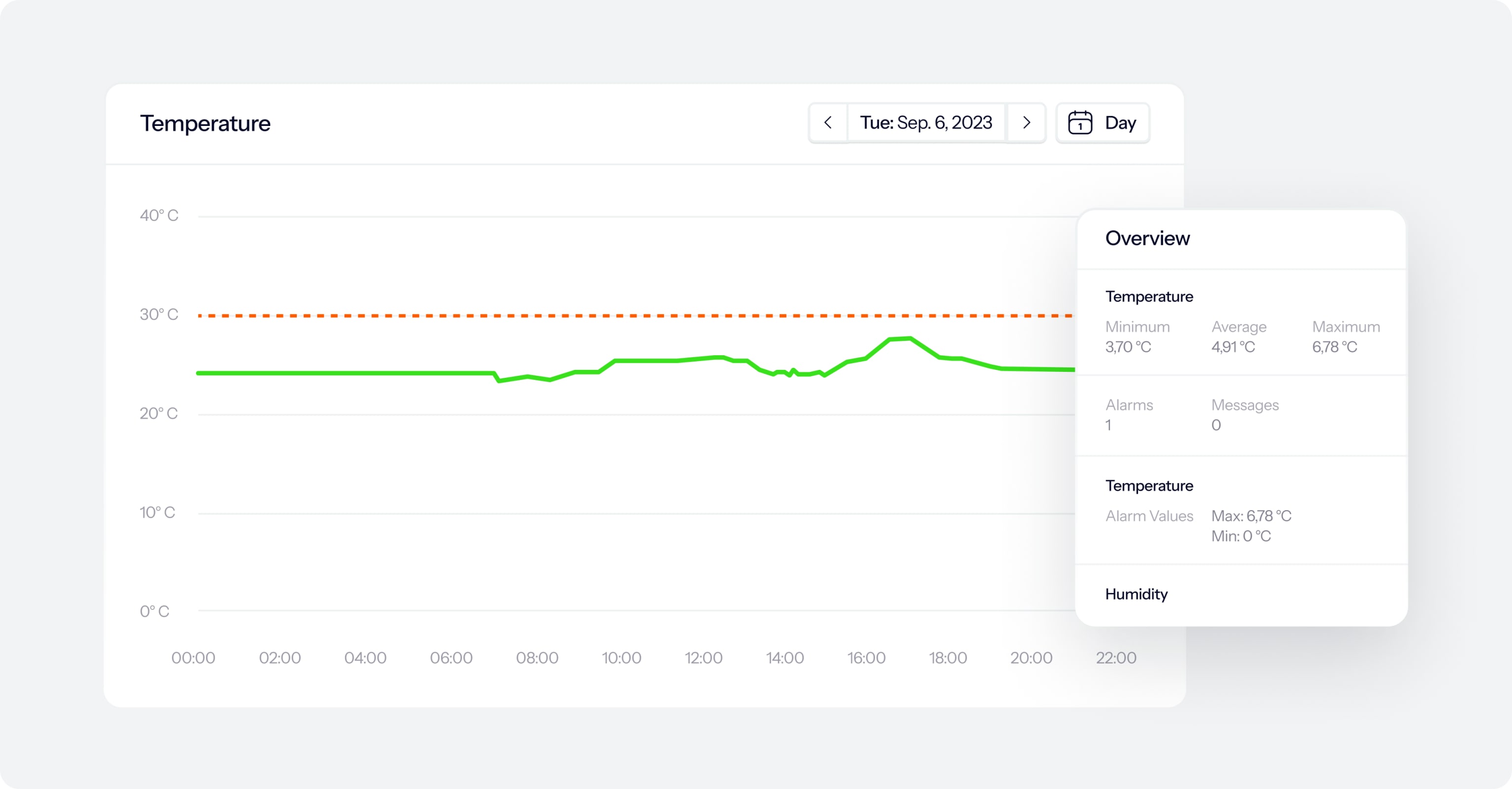
How Eupry’s remote temperature monitoring system works
Stop spending valuable hours searching for data. We have collected every data point you need in our temperature compliance platform.
Watch real-time and historic measurements in a user-friendly, IT-validated platform that holds all your temperature compliance data – from calibration certificates to alarms, deviation reports, mapping data, and more.
See examples of what you can access on the platform:

Talk to a specialist
Do you have a questions, need a quote, or just looking for more information? We are here to help.
Let us know what you are looking for, and we will send the information – or set up a talk.
Technical specifications of the data loggers
Find the equipment that fits your needs. See more details and options in our product catalog.

FAQ: Eupry’s remote temperature monitoring solution
One vendor, one solution
All you need for thermal compliance
Eupry brings temperature and humidity compliance – monitoring, calibration, and mapping – into one digital, GxP-compliant solution, providing CONTINUOUS compliance and one centralized overview.






