How to make your temperature monitoring audit-ready
Best practices for GxP environments
Temperature monitoring is a core part of any temperature compliance audit and, unfortunately, a common source of findings. Your monitoring setup should link product knowledge, risk assessments, and documented decisions. Here is how to meet auditor expectations.

When audit issues emerge for temperature monitoring, it is often not because organizations lack systems and processes, but because the rationale linking product knowledge, risk, and monitoring decisions is not documented or understood.
This guide outlines what auditors typically expect to see in your monitoring setup, from placement rationales to alarm strategies and calibration practices. Use it to identify potential gaps in your monitoring practices.
Also read: How to master temperature monitoring in critical environments
This guide covers:
- Placement rationale
- Alarm configurations
- Calibration of monitoring data loggers
- Monitoring system and data integrity
- System roles and responsibilities
- Supporting procedures
- Documentation
- FAQ about audit-ready monitoring
Also read: Complete guide: What auditors expect from your temperature compliance program

Note!
This guide offers general guidance only and does not guarantee audit outcomes or regulatory compliance. All recommendations should be evaluated in the context of your specific processes and risks.
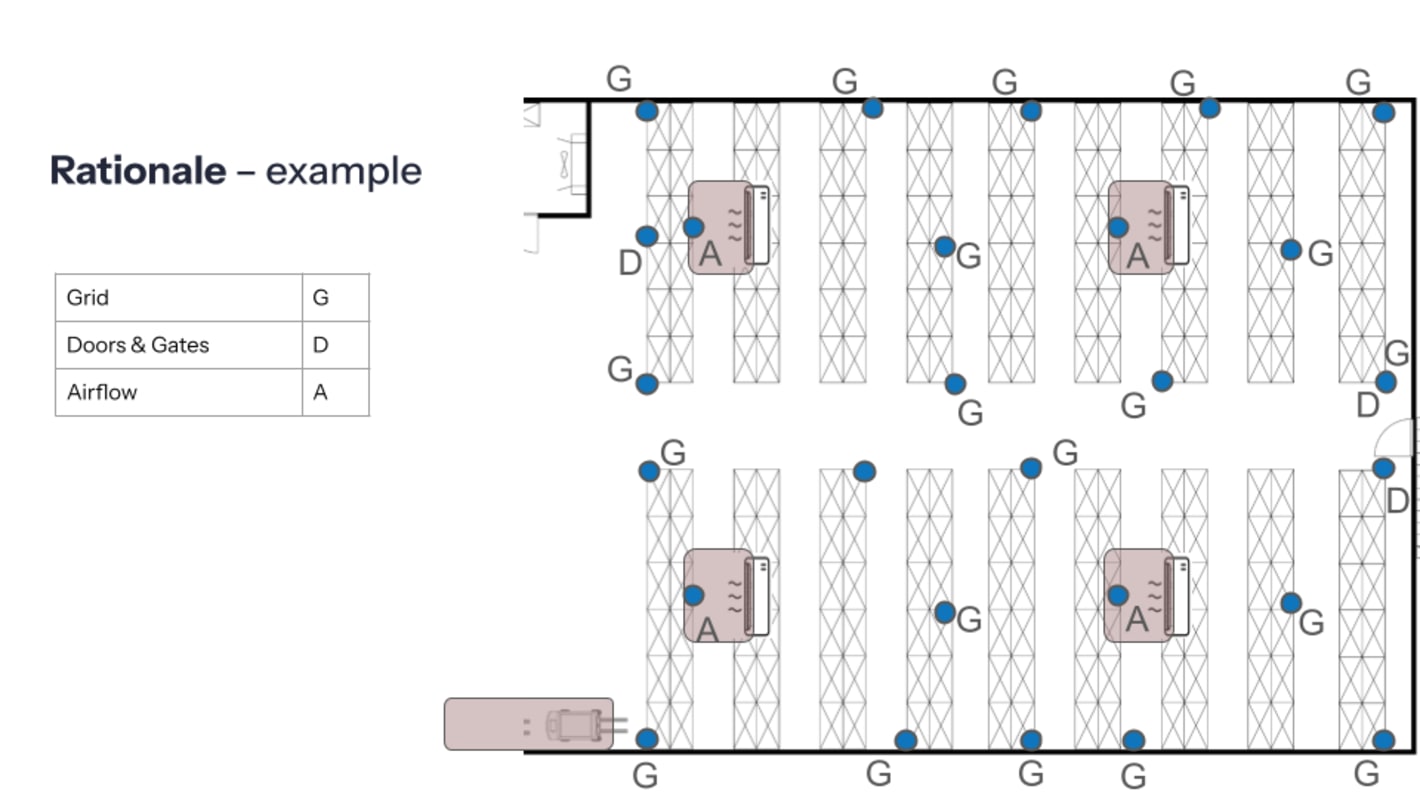
Placement rationale
Auditors may start by pointing to a logger and asking: “Why is this placed here?” Understanding and documenting your placement rationale for data loggers is essential. Your answer should be grounded in mapping results, risk assessments, and the criticality of what is stored nearby.
Some of the key elements to have ready during an audit are:
- Written rationale for each sensor location
- References to mapping results showing hot and cold spots
- Consideration of risk, such as proximity to doors, vents, or heat sources
- Alignment with product sensitivity and storage requirements
- Version-controlled floor plans or schematics
Tip
Make sure all your monitoring configurations are traceable to risk. Your logger placements, alarm configurations, and calibration strategy should all reflect your risk assessments and temperature mapping results.
Alarm configurations
Alarm limits are important, and auditors will most likely expect you to justify the thresholds, delays, and settings based on product and process risk, not convenience.
Key monitoring alarms documentation to have ready for audits includes:
- Defined alarm limits with documented rationale
- Time delays linked to product stability and excursion definitions
- Clearly separated criteria for non-conformities and insignificant alarms
- Records showing who configured alarms and when
- SOPs for alarm response that reflect actual practice
Calibration of monitoring data loggers
Calibration is about proving accuracy, documenting how it is maintained, and showing how these choices are based on risk – a field that your auditors will almost always check to secure your temperatur sensors can be trusted, not just at the point of installation, but throughout their use.
Your calibration strategy should be documented and justifiable. Avoid blindly trusting supplier suggestions. While these might be useful and qualified, it is important that you understand and can explain why your approach fits your risk profile and requirements.
Key calibration elements to have ready during an audit include:
- Calibration certificates for all in-use sensors, current, and traceable
- Defined calibration intervals based on risk, not default values
- Rationale for using accredited vs traceable calibration methods
- Calibration tolerances aligned with product and process requirements
- Change records if intervals, tolerances, or methods were updated
- SOPs covering how calibration results are reviewed and acted upon
Also read: 8 temperature calibration components you should have ready for audits

Monitoring system and data integrity
Auditors will assess how your monitoring system handles audit trails, backup, access control, and data retention to support data integrity. Be prepared to show how the system ensures secure records, traceability, and long-term retention.
Key system features to document:
- How audit trails are maintained and reviewed
- Access control policies and user permissions
- Where data is the data stored
- Data backup frequency and retention settings
- Evidence of system validation and change managemen
System roles and responsibilities
Monitoring systems often span multiple teams, which is fine, but the responsibilities have to be clearly assigned. Auditors will often want to know who does what and how you make sure everyone is aligned on this role distribution.
Responsibility documentation to have ready for an audit:
- Documented role assignments for monitoring-related tasks
- Training records for everyone with access to the system
- Audit trail access and review procedures
- Defined responsibilities for updates, alarms, and maintenance
- Internal procedures for managing system changes
Tip: Also consider system and process validation roles. Understand which validation responsibilities your supplier holds and which fall to you, and document this split clearly.

Supporting procedures
Your monitoring setup is only as strong as the procedures that sustain it. Your auditors will want to see that your processes for setup, maintenance, alarm response, etc., are well-defined and followed. Make sure your procedures reflect reality, and that records show the process is being followed.
Key operating procedures to document for audits:
- Setup and installation of data loggers
- Alarm response workflows and escalation steps
- Sensor calibration and certificate review
- Excursion handling and deviation documentation
- Routine checks and system maintenance
- Procedures for training and re-training relevant staff
Documentation (that shows control)
Successful audits are dependent on clarity and traceability, and this is best secured through thorough documentation. Focus on documenting not just what you do, but why you do it. The clearer the link between your process understanding and your monitoring setup is documented, the easier it is for auditors to assess your compliance and the credibility of your procedures.
Some of the most important elements to document are:
- Sensor placement rationale tied to mapping and risk
- Alarm limits and delay settings with justification
- Calibration strategy, intervals, and tolerances
- Role assignments and access permissions
- Change records for system updates or reconfigurations


Checklist: Audit-ready temperature monitoring in GDP
What do auditors look for in your temperature monitoring (as well as mapping and calibration) processes in GDP? Download the checklist based on GDP guidelines.

Checklist: Temperature monitoring audit checklist for GMP
Get the free checklist covering the areas auditors often look at during audits in GMP.
FAQ about audit-ready monitoring
Often asked questions about audit-ready temperature monitoring.
Learn more
On-demand webinar: Audit-ready temperature compliance
Learn how to get your GxP temperature monitoring and mapping audit-ready, avoid frequent errors, and reduce prep time.

Guide: How to prepare your temperature mapping processes for audits
Audits can be stressful. How do you ensure your thermal MAPPING processes stand up to inspection?

Solution: Automated temperature monitoring for GMP and GDP
Manual monitoring is error-prone. Free time and minimize quality risks with one digital, automated, and wireless monitoring system.