Temperature monitoring and alarms in pharma cold rooms
Temperature monitoring and alarms in pharmaceutical cold rooms are key parts of securing GxP compliance. Missed alarms, poor documentation, or calibration issues can cause deviations and repeat work. This guide explains how to efficiently design compliant monitoring procedures for cold storage, manage alarms, and document deviations.
Verify your setup agains GxP requirements.
Why is temperature monitoring critical in pharma cold storage?
Continuous monitoring and reliable alarms are essential for ensuring that pharmaceutical cold storage rooms remain compliant with GDP and GMP requirements. Monitoring ensures that medicinal products remain within labeled conditions throughout storage. It provides documented proof for inspectors and protects patients from compromised medicines. Without reliable monitoring, companies risk undetected excursions, regulatory findings, and costly product losses.
Also read: Pharmaceutical cold storage: Applications and GDP/GMP requirements
How should monitoring systems be designed for cold storage?
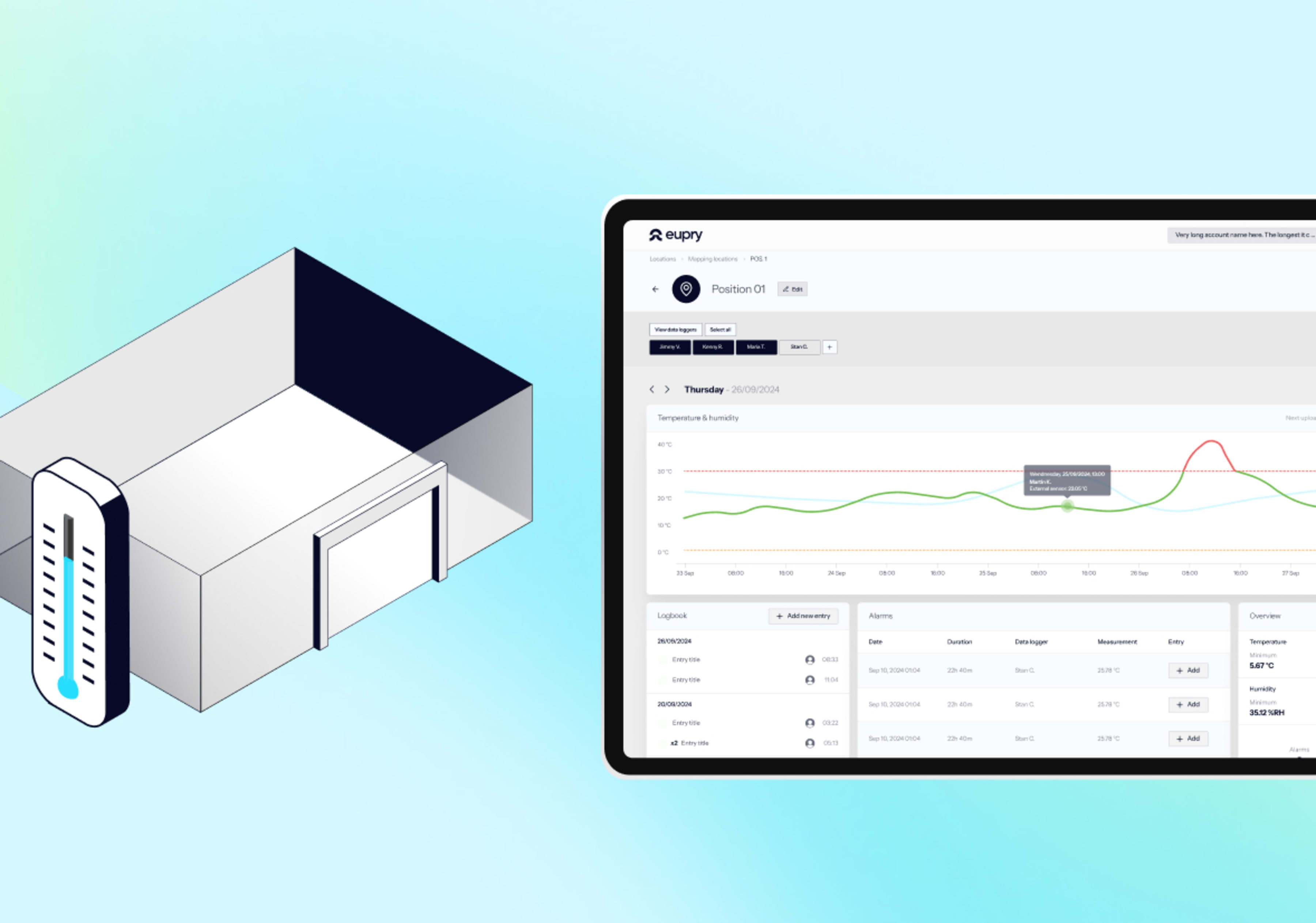
Monitoring systems must be designed to capture real conditions, reflect mapping results, and ensure secure data. Regulators emphasize accuracy, traceability, and proper placement of permanent sensors.
Core requirements for monitoring system design include:
- Sensor placement: Install sensors at hot and cold spots identified by temperature mapping.
- Calibration requirements: Use calibrated data loggers with certificates traceable to national or international standards.
- Continuous data logging: Record conditions around the clock with intervals appropriate for the risk level.
- Data integrity and security: Store records in validated systems with audit trails, following Annex 11 and 21 CFR Part 11.
Also read: The ultimate temperature mapping guide
How should alarms be managed in cold storage rooms?
Alarms are only effective if they are configured correctly and followed by timely actions. Regulators expect companies to test alarms, document responses, and define clear escalation paths.
Best practices for alarm management include:
- Defined setpoints: Establish alert and action limits in line with product label requirements and risk assessments.
- Regular alarm testing: Perform challenge tests for high, low, and communication failures.
- Escalation process: Define responsibilities and timelines for responses.
- Alarm documentation: Record acknowledgments, actions taken, and final resolutions.
Also read: Audit-ready cold room monitoring checklist (alarms, logs, deviations)

Download a cold room monitoring checklist [alarms, logs, deviations]
Ensure your cold storage monitoring setup meets every GDP and GMP requirement
What are the best practices for deviation handling in monitoring cold storage?
Deviations must always be recorded, assessed for product impact, and resolved with corrective and preventive actions (CAPAs). Regulators expect a structured approach to avoid repeated issues.
Deviations must always be documented thoroughly. Each event should include details such as cause, duration, and corrective actions taken. Where relevant, excursions must be evaluated using mean kinetic temperature (USP <1079.2>) to assess potential product impact. CAPAs should then be assigned, documented, and closed in a timely manner to prevent recurrence. Finally, all deviation records should be subject to QA review and approval before closure, ensuring compliance and audit readiness.
Also read: Pharmaceutical cold room qualification (IQ/OQ/PQ) and temperature mapping
How often should monitoring systems be reviewed and requalified?
Monitoring systems should be periodically reviewed to confirm ongoing compliance. Regulators expect risk-based requalification and documented reviews.
Review and requalification activities include:
- Calibration intervals: Define based on criticality, risk, and manufacturer recommendations.
- System requalification: Perform after major changes, upgrades, or failures.
- Audit trail reviews: Conduct and document periodic reviews of electronic records.
- Management oversight: Trend alarms and deviations to identify systemic issues.
Also read: How to master temperature monitoring in GxP
FAQ about temperature monitoring in pharma cold storage rooms
Cold room temperature monitoring checklist [alarms, logs, deviations]
Get instant access to a structured tool to verify that your monitoring setup is audit-ready, aligned with USP <1079>, Annex 11, and 21 CFR Part 11 requirements.
- Sensor placement and calibration
- Alarm and deviation management
- Reporting and review

Cold room monitoring system for GDP and GMP
Cold room monitoring made simple. Keep your pharma facilities compliant and under control – without manual work.
- Instant SMS/e-mail alerts: Stop deviations before they cause issues.
- 3-click audit reports: GxP/ISO 17025/Part 11 documentation in clicks.
- Central control: Get one digital overview across sites and units.
- Reliable sensors: Built for cold rooms and ultra-low environments.
