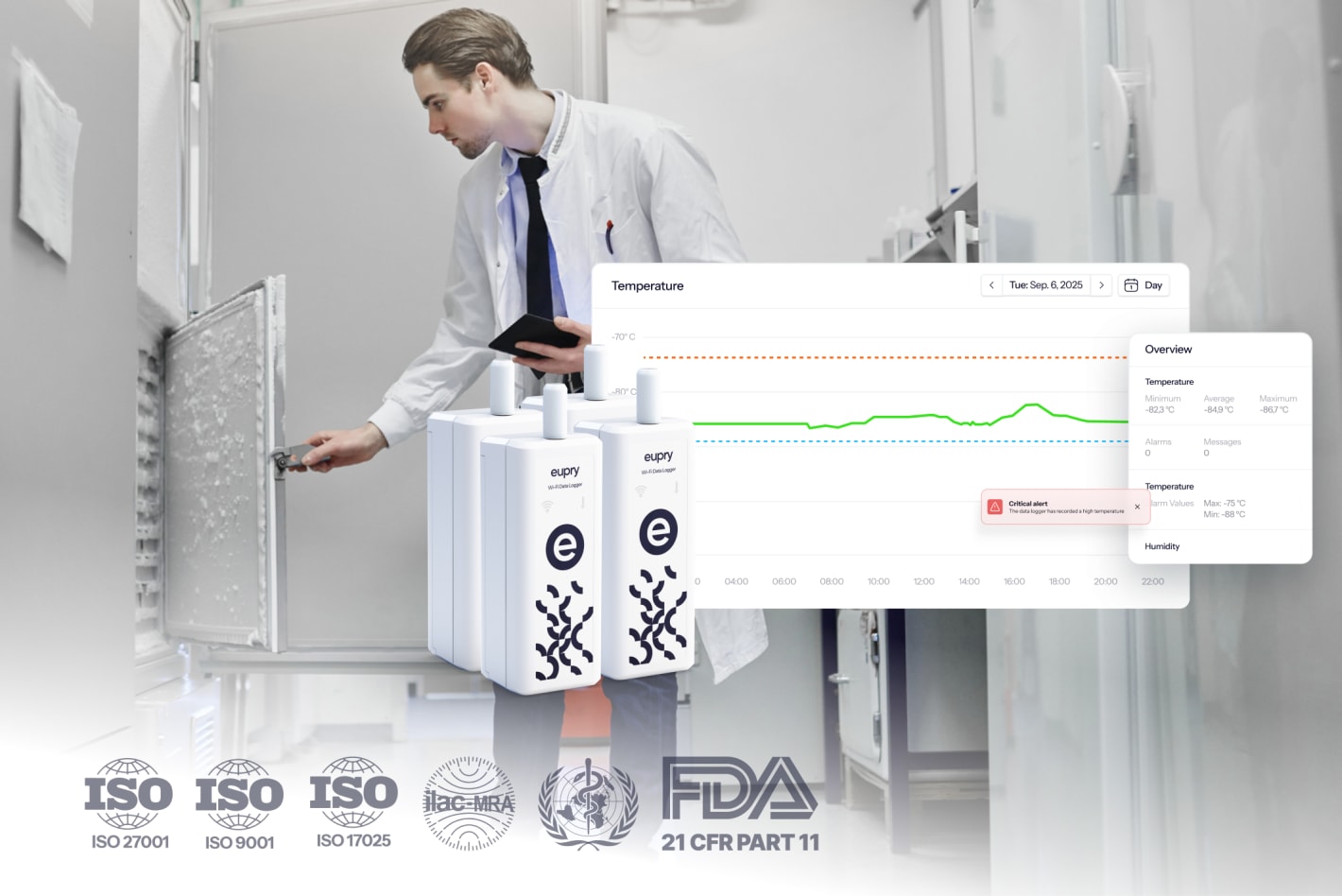
Temperature-controlled units (TCUs) in pharma
Qualification, mapping, and monitoring guide

Adam Hartmann-Kruckow
Learn best practices for qualifying, mapping, and monitoring temperature-controlled units (TCUs) in pharma, biotech, and healthcare logistics and meet GDP and GMP requirements
Download our step-by-step checklist covering qualification, mapping, monitoring, and calibration for all TCU types.
Note:
This guide is provided for general educational purposes only and does not replace official regulatory requirements or professional judgment and the templates offered here are examples and must be adapted, reviewed, and approved within your company’s quality management system before use.
Managing temperature-sensitive products in pharma and biotech is not optional. It is the foundation of compliance, product integrity, and patient safety.
But the challenge goes beyond basic temperature control. Your refrigerators, freezers, incubators, and cold rooms need documented proof that they work correctly, consistently, and under the conditions they will face every day. That means qualification, mapping, monitoring, and calibration according to Good Distribution Practice (GDP), Good Manufacturing Practice (GMP), and a stack of other requirements.
Whether you are validating a new unit, preparing for an audit, or standardizing compliance across sites, this guide provides the practical answers you need.
What is a "temperature-controlled unit"?
A temperature-controlled unit – or TCU – is any equipment used to store or transport products within a defined temperature range. In pharma and biotech, these units protect medicinal products, biologics, vaccines, and active pharmaceutical ingredients from temperature excursions that could compromise quality or safety.
Regulatory definitions come from GDP guidelines, GMP Annex 15, and standards like USP <1079> and WHO TRS 961. The common thread: if your product needs temperature control, your equipment needs to prove it can deliver that control reliably.
What equipment types are considered TCUs?
Temperature-controlled units include several equipment categories, each serving specific storage needs:
- Refrigerators and pharmacy fridges: Store products between 2°C and 8°C. Commonly used for vaccines, insulin, and biologics that require cold chain protection.
- Freezers: Operate between -15°C and -25°C for long-term storage of biologics and some active pharmaceutical ingredients.
- Ultra-low temperature (ULT) freezers: Maintain ultra low temperatures, often around -80°C and used for cell therapies, gene therapies, and sensitive biologics requiring deep-freeze conditions.
- Incubators: Control temperature (and sometimes humidity) for stability testing, microbiology work, and cell culture applications.
Also read: Pharmaceutical refrigerators: validation, mapping and monitoring
Get a step-by-step guide to qualifying, mapping, and monitoring temperature-controlled units in pharma

Which standards apply to temperature-controlled units?
Multiple regulatory frameworks govern how you qualify and monitor temperature-controlled units. Understanding which apply to your operation helps you prioritize compliance efforts:
GDP (Good Distribution Practice): Covers storage and transport of medicinal products, including temperature mapping, continuous monitoring, and alarm requirements for distribution operations.
GMP Annex 15: Focuses on qualification and validation of equipment used in manufacturing, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
USP <1079>: Provides guidance on storage and shipment of temperature-sensitive products, including Mean Kinetic Temperature (MKT) calculations for excursion evaluation.
21 CFR Part 11: Sets requirements for electronic records and electronic signatures. Relevant if you use digital monitoring systems or computerized validation tools.
WHO TRS 961 (Annex 9): Offers globally recognized guidance on temperature mapping and monitoring for pharmaceutical storage areas.
ISO 21973: Addresses temperature-controlled transport for pharmaceutical products in the supply chain.
Why do temperature-controlled units need qualification?
Unqualified equipment puts your products at risk. Temperature excursions can degrade active ingredients, reduce efficacy, or render products unsafe for patients. Beyond product quality, unqualified TCUs create audit risks that can lead to regulatory observations or warnings.
Inspectors expect to see documented evidence that your equipment works as intended and keeps working over time. Proper qualification protects product quality, supports patient safety, and keeps you audit-ready when inspectors arrive.
How do you validate a temperature-controlled unit?
Validating a temperature-controlled unit means proving it does what it's supposed to do, consistently and reliably. This process follows three phases: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Each phase builds on the previous one, creating a documented trail that shows your unit meets specifications under real-world conditions.
Also see: IQ, OQ, PQ in pharmaceuticals: Complete guide to equipment qualification and compliance

Temperature mapping of temperature-controlled units
When do you need to conduct mapping of a temperature-controlled unit?
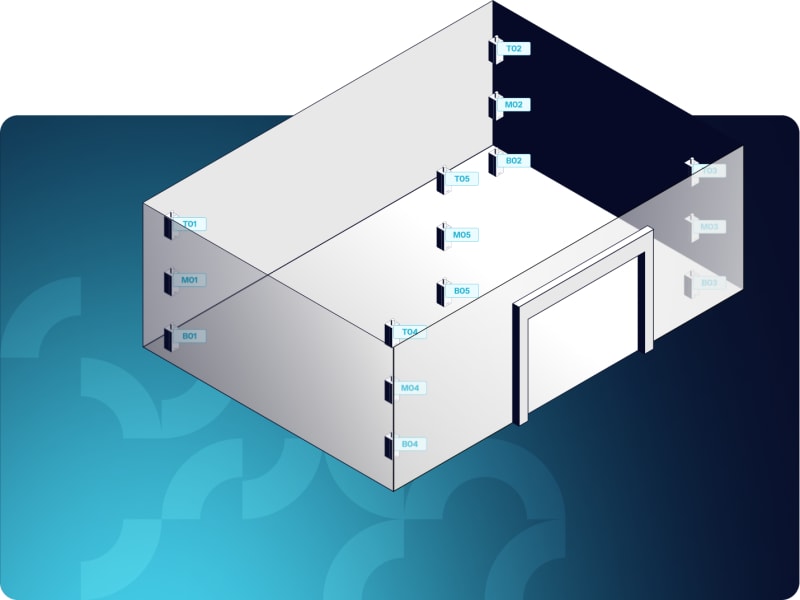
Mapping proves that your temperature-controlled unit maintains uniform temperature distribution throughout its storage space. It identifies hot and cold spots, validates sensor placement, and provides the data you need to determine where to position permanent monitoring sensors.
Temperature mapping is required during initial qualification (as part of OQ or PQ) and periodically thereafter, or whenever conditions change that could affect temperature distribution.
Why is temperature mapping required for TCUs?
Regulations under GDP, GMP, and WHO guidelines require mapping to demonstrate that your storage area maintains the required temperature range across all locations where product is stored.
Mapping answers critical questions that visual inspection cannot:
- Where are the hot and cold spots in this unit?
- Do all areas stay within acceptance criteria under normal conditions?
- Where should you place permanent monitoring sensors to catch deviations?
- How does the unit respond to door openings, product loading, or other disruptions?
Without mapping, you are guessing about temperature distribution. Mapping gives you data-driven confidence.
How do you determine sensor placement and mapping frequency?
Not all TCUs require the same mapping approach. Risk-based thinking helps you determine how many sensors you need, where to place them, and how often to re-map.
Factors that influence your mapping approach:
- Product criticality: High-value biologics or temperature-sensitive vaccines require more rigorous mapping than stable chemical products with wider acceptable temperature ranges.
- Unit size and complexity: A small countertop refrigerator needs fewer sensors than a 1,000-square-meter warehouse with multiple zones and varying airflow patterns.
- Historical performance: Units with a history of stable performance may qualify for extended re-mapping intervals if you can justify the approach.
- Regulatory expectations: Some facilities require annual re-mapping based on local inspector preferences. Others accept continuous mapping as an alternative if properly validated.
Also read: Guidelines for risk-based temperature mapping in GxP
What goes into a mapping protocol for TCUs?
A good mapping protocol defines acceptance criteria, sensor placement, test duration, and challenge tests before you start. It should be written in advance and approved by quality assurance.
Key elements of a mapping protocol:
- Acceptance criteria (for example, ±2°C from setpoint for all sensor locations)
- Number and placement of sensors (based on grid approach or risk-based assessment)
- Mapping duration (typically 24 to 72 hours for small units, longer for large areas or seasonal validation)
- Challenge tests (door-open recovery, product loading impact, seasonal conditions if applicable)
- Data analysis methods (identifying hot and cold spots, calculating MKT if needed for your application)
Your protocol is your roadmap. It tells you what to do, how to do it, and what success looks like.
Also read: How to create a mapping protocol

Temperature mapping protocol for freezers/ULTs
This document is your blueprint for protocol design, covering objectives, acceptance criteria, sensor strategy, test conditions, and reporting expectations. Use the template to accelerate drafting, reduce deviations, and align with GxP expectations.
When should you re-map versus use continuous mapping?
Traditional mapping requires periodic re-qualification, typically annually or after any significant change (new equipment installation, layout modifications, HVAC system upgrades, or changes in product loading patterns).
Continuous mapping offers an alternative approach. Instead of repeating full mapping studies on a fixed schedule, you validate that your permanent monitoring system provides continuous evidence of temperature distribution. This approach reduces downtime, cuts costs, and keeps you compliant without disrupting operations.
Also read: Continuous temperature mapping: A framework to eliminate re-mapping
Equipment-specific guidance
Different TCU types have unique mapping considerations based on their size, configuration, and typical use patterns:
- Refrigerators: How to map pharma fridges for GDP, GMP, and USP compliance
- Freezers: Freezer temperature mapping guidelines
- Cold rooms: Pharma cold room IQ/OQ/PQ and mapping
- Warehouses: Temperature mapping for GDP pharmaceutical warehouses

Download a TCU temperature compliance checklist
Get the full step-by-step checklist covering qualification, mapping, monitoring, and calibration.
Temperature monitoring of temperature-controlled units
How do you monitor TCUs in pharma?
Once your TCU is qualified and mapped, you need continuous temperature monitoring to prove it stays within specification during routine use. Monitoring systems track temperature in real time, trigger alarms if conditions deviate, and generate the audit trails you need for regulatory compliance.
Monitoring means your system checks the temperature at defined intervals (typically every 1 to 15 minutes depending on risk) and stores data electronically.
But not all monitoring systems meet regulatory requirements. Annex 11 and 21 CFR Part 11 set strict standards for electronic records, data integrity, and system validation that manual logbooks cannot satisfy.

How should you configure alarms for monitoring of TCUs?
Alarms are your early warning system. They alert you to problems before they become full excursions that put product at risk.
But poorly configured alarms create their own problems. Too many false alarms lead to alarm fatigue where staff stop responding. Too few alarms mean you miss critical events until it is too late.
A good alarm strategy includes three components:
- Setpoints: Define the temperature threshold that triggers an alarm. For example, 2°C to 8°C ±2°C for a pharmacy fridge means alarms at 0°C and 10°C.
- Delays: Build in a short delay (typically 15 to 30 minutes) to avoid nuisance alarms from brief door openings that don't threaten product.
- Escalation: Route alarms to the right people at the right time. On-site staff during business hours, on-call personnel after hours, and quality assurance for critical deviations.
Also read: GDP monitoring requirements: how to meet Annex 11, 21 CFR Part 11, and alarm rules
What are audit trail and e-signature requirements for monitoring?
Annex 11 and 21 CFR Part 11 require that electronic systems maintain secure, time-stamped audit trails for all data modifications. If someone changes a setpoint, edits a temperature record, or acknowledges an alarm, the system must log who did it, when they did it, and why.
E-signatures provide an additional layer of accountability. Critical actions like approving a deviation investigation or releasing a batch after an excursion require documented authorization that meets regulatory standards.
How do you maintain data integrity (ALCOA+)?
ALCOA+ is the foundation of data integrity in regulated industries. Your monitoring system must support these principles:
- Attributable: Every action must be linked to a specific user with unique login credentials.
- Legible: Data must be readable and understandable to reviewers and inspectors.
- Contemporaneous: Data must be recorded at the time the event occurs, not retrospectively.
- Original: Keep the original record or a verified true copy with documented procedures.
- Accurate: Data must be correct and free from errors or unauthorized changes.
- Complete: All relevant data must be captured without selective deletion.
- Consistent: Data must be recorded consistently across systems and over time.
- Enduring: Records must be stored securely and remain accessible throughout the retention period.
- Available: Data must be retrievable for review, audit, or inspection when needed.
Modern monitoring systems build ALCOA+ compliance into their design, reducing the risk of data integrity failures that can trigger regulatory observations.
Calibration of sensors for temperature-controlled units
How often do you need to calibrate TCU sensors?
Calibration proves that your temperature sensors are accurate. Over time, sensors drift away from true readings. Calibration corrects that drift and provides documented evidence that your measurements are traceable to national or international standards.
ISO 17025-accreditation is the gold standard for calibration. It demonstrates that your calibration provider operates under a quality management system audited by an independent accreditation body.
Also read: ISO 17025 in temperature compliance: What you need to know

Why is calibration required for temperature-controlled units?
Regulators expect calibrated sensors because uncalibrated sensors cannot be trusted. Without calibration, you can't prove your temperature data is accurate. And if your data is not accurate, your entire compliance strategy is built on unreliable information.
Calibration answers a simple but critical question: Does this sensor measure what it's supposed to measure?
How do you determine calibration frequency for your TCU's temperature sensors?
Most organizations calibrate sensors annually as a default. But frequency should be based on risk, not arbitrary timelines. High-value products, unstable equipment, or sensors in harsh environments may require more frequent calibration.
Factors that influence calibration frequency:
- Sensor type and quality (higher-quality sensors with proven stability drift less over time)
- Environmental conditions (extreme temperatures or humidity levels accelerate drift)
- Historical calibration data (consistent performance with minimal drift may justify extended intervals)
- Product criticality (biologics with narrow acceptable ranges justify more frequent calibration than stable chemicals)
What is the difference between on-the-wall and traditional calibration?
Traditional calibration requires you to remove sensors, send them to a calibration lab, and wait for results. During that time, you either lose monitoring coverage or swap in temporary sensors. Both options create gaps in your data and add work for your team.
On-the-wall calibration changes the process completely. Instead of removing sensors, a reference probe visits each sensor at its installed location. The system compares the sensor reading to the reference, calculates any offset, and applies a correction. All in minutes, without removing anything or disrupting monitoring.
Also read: What is on-the-wall calibration?
How do you handle temperature excursions in TCUs?
Temperature excursions happen. Equipment fails, doors get left open, and HVAC systems malfunction. The question isn't whether excursions will occur – it's how you handle them when they do.
USP <1079.2> provides a framework for evaluating excursions using Mean Kinetic Temperature (MKT). MKT calculates the single temperature that would produce the same degradation as a series of fluctuating temperatures over time.
What process should you follow for excursion investigations?
When an excursion occurs, you need a documented process for investigation, impact assessment, and corrective action. Regulators expect to see evidence that you took appropriate steps to protect product quality.
Steps in excursion handling:
- Identify the affected product batches and determine the duration and severity of the excursion
- Assess the impact using MKT calculations or other risk-based methods appropriate for your product
- Determine whether the product can be released for use, requires reworking, or must be destroyed
- Document the investigation thoroughly and obtain quality assurance approval for the disposition decision
- Implement corrective and preventive actions (CAPA) to reduce the risk of future excursions
Also read: How to investigate a temperature excursion or deviation faster
When is MKT calculation acceptable?
MKT isn't appropriate for every situation. It works for chemical degradation that follows Arrhenius kinetics, but it doesn't account for physical changes like freezing, crystallization, or protein denaturation.
Use MKT when:
- The product's degradation pathway follows predictable chemical kinetics
- The excursion involves moderate temperature fluctuations within a reasonable range
- You have stability data that supports MKT-based decision-making for your specific product
Don't use MKT when:
- The product experienced freezing or extreme heat exposure
- Physical changes like protein aggregation or crystal formation may have occurred
- You lack stability data to validate the MKT approach for your product type
Also read: USP <1079.2> explained: 7 updates for excursion evaluation in GxP
What documentation do you need for excursions?
Every excursion needs a complete paper trail. Inspectors expect to see documented evidence that you identified the problem, assessed the risk to product quality, made a defensible decision about product disposition, and took steps to prevent recurrence.
Required documentation:
- Temperature data showing the exact time, duration, and severity of the excursion
- Impact assessment (including MKT calculation if applicable to your situation)
- Product disposition decision with clear rationale (release, quarantine, or reject)
- Root cause analysis identifying why the excursion occurred
- Corrective and preventive actions (CAPA) to address the root cause

Download a temperature excursion investigation template
Download a free template based on GxP best practices and get a practical tool to structure your excursion investigation.
How do you standardize TCU thermal compliance across multiple sites?
If you operate multiple facilities, you face a challenge: how do you maintain consistent temperature compliance across sites while respecting local differences in equipment, climate, and regulatory expectations?
Harmonization does not mean forcing every site to do things exactly the same way. It means creating a common framework – standard operating procedures (SOPs), unified acceptance criteria, and centralized oversight – while allowing flexibility where local conditions require it.
How do you create unified SOPs across facilities?
Start with a master SOP that defines the overall approach to temperature qualification, mapping, monitoring, and calibration. Then allow site-specific appendices that address local conditions like climate differences, equipment variations, or local regulatory requirements.
Elements of a unified SOP:
- Acceptance criteria for temperature ranges that apply across all sites
- Mapping frequency and protocol structure with flexibility for site-specific conditions
- Alarm setpoints and escalation procedures adapted to local staffing patterns
- Calibration frequency and methods standardized where possible
- Documentation and reporting requirements consistent across the organization
What role do Validation Master Plans play for TCUs?
A Validation Master Plan provides a high-level strategy for all validation activities across your organization. It defines roles, responsibilities, and the scope of validation for each facility type.
For temperature compliance, your VMP should address:
- Which equipment types require validation at each facility
- How often re-qualification is needed based on risk assessment
- Who approves protocols and reports at each organizational level
- How you manage change control for new equipment, facility expansions, or process changes
How do you define role-based responsibilities?
Clear roles prevent confusion and create accountability for temperature compliance activities. Each role needs defined responsibilities that fit within the larger quality system.
Typical roles in temperature compliance:
- Quality assurance (QA): Approves protocols before execution, reviews validation reports, and confirms compliance with SOPs and regulatory requirements.
- Facility managers: Oversee day-to-day monitoring, respond to alarms, coordinate maintenance activities, and manage local compliance documentation.
- Validation engineers: Design and execute mapping studies, analyze temperature data, write validation reports, and support deviation investigations.
- Calibration teams: Perform sensor calibration according to schedule, maintain calibration records, and manage calibration equipment.
Defining these roles upfront avoids gaps where nobody takes ownership and overlaps where multiple people duplicate work.
Frequently asked questions about temperature-controlled units
Learn more
Bring monitoring, mapping, and calibration into ONE solution
Managing TCUs across qualification, mapping, monitoring, and calibration doesn't have to be complicated. Eupry brings everything into one platform, giving you full control and audit-ready data.