Pharmaceutical cold chain monitoring

Adam Hartmann-Kruckow
Get a full overview of everything you need to know about pharmaceutical cold chain monitoring – why it is required, how it works, and which requirements you need to live up to in GxP.
Get a structured framework to assess vendors for GxP cold chain monitoring systems.

Introduction
Cold chain monitoring tracks temperature conditions in real time across pharmaceutical storage and distribution to prevent product degradation and ensure GxP compliance. This guide explains when monitoring is required, how to implement continuous monitoring systems, and what regulators expect from your cold chain management program.
The pharmaceutical industry loses an estimated $35 billion annually to cold chain failures. Monitoring provides the visibility needed to prevent excursions, protect product integrity, and maintain audit readiness across your supply chain.
Also see: How to select cold chain monitoring devices and sensors
Table of contents
- What is cold chain monitoring in pharmaceuticals?
- Why is cold chain monitoring required?
- How does cold chain monitoring work?
- Where is cold chain monitoring used in pharmaceuticals?
- What are the key components of a cold chain monitoring system?
- What are the GxP requirements for cold chain monitoring?
- How do you select a cold chain monitoring system?
- FAQ about pharmaceutical cold chain monitoring

What is cold chain monitoring in pharmaceuticals?
Cold chain monitoring is the continuous or periodic measurement and documentation of temperature conditions for pharmaceutical products during storage and transport. It ensures products remain within their validated temperature ranges from manufacturing through to the patient.
For temperature-sensitive biologics, vaccines, and specialty drugs, monitoring serves three critical functions. It provides real-time visibility into storage conditions across warehouses, refrigerators, freezers, and transport vehicles. It creates the documented evidence required for GDP compliance and regulatory inspections. And it enables immediate response when deviations occur, reducing the risk of product loss.
Modern monitoring systems combine wireless sensors, cloud-based software, and automated alerts to replace manual logging processes. This shift from reactive documentation to proactive control represents a fundamental change in how pharmaceutical companies manage cold chain compliance.
Also read: Temperature monitoring guide for pharma
Why is cold chain temperature monitoring required?
Regulatory bodies worldwide mandate temperature monitoring wherever product quality depends on controlled conditions. EU GDP Guidelines, WHO TRS 961, USP 1079 series, and FDA regulations all require documented evidence that storage and transport environments remain within specified limits.
The requirement stems from a simple principle: temperature excursions compromise drug efficacy and patient safety. Insulin loses potency when exposed to heat. Vaccines become ineffective after freezing. Biologics degrade when temperature ranges are exceeded. Without monitoring, these failures go undetected until products reach patients.
These requirements extend throughout the entire supply chain. Manufacturers must monitor during production and storage. Distributors need continuous oversight during transport. Healthcare facilities must document conditions until administration. Each handoff point represents a compliance obligation and a potential failure mode.
Beyond regulatory compliance, monitoring reduces financial risk. Temperature-related product losses, recalls, and regulatory findings create costs that far exceed monitoring system investments. Real-time visibility prevents these losses by enabling intervention before products are compromised.

Get a cold chain monitoring system selection checklist
Get a structured framework to assess vendors for cold chain monitoring systems in GxP.
How does cold chain monitoring work?
Cold chain monitoring combines three elements: sensors that measure conditions, data loggers that record and transmit readings, and software platforms that analyze data and generate alerts.
Sensors and measurement
Temperature sensors are placed in critical locations within refrigerators, freezers, warehouses, and transport vehicles.
Modern wireless sensors measure conditions continuously, typically at intervals between one and five minutes. Sensor accuracy is critical for GxP compliance. Pharmaceutical monitoring requires measurement accuracy of ±0.5°C or better, with ISO 17025 accredited calibration certificates documenting traceability to national standards.
Placement strategy determines monitoring effectiveness. Risk-based sensor deployment focuses coverage on hot spots, cold spots, door areas, and product storage zones identified through initial temperature mapping studies.
Learn more about choosing cold chain monitoring devices and sensors.
Data collection and transmission
Data loggers collect sensor readings and transmit them to cloud platforms via Wi-Fi or cellular networks. This eliminates manual data downloads and ensures continuous visibility even when staff are offsite. Modern systems buffer data locally during connectivity gaps, then sync automatically when networks restore.
Remote temperature monitoring enables teams to view live conditions across multiple sites from any device. Centralized dashboards provide real-time status, historical trends, and compliance metrics for entire facility networks.
Alert management and response
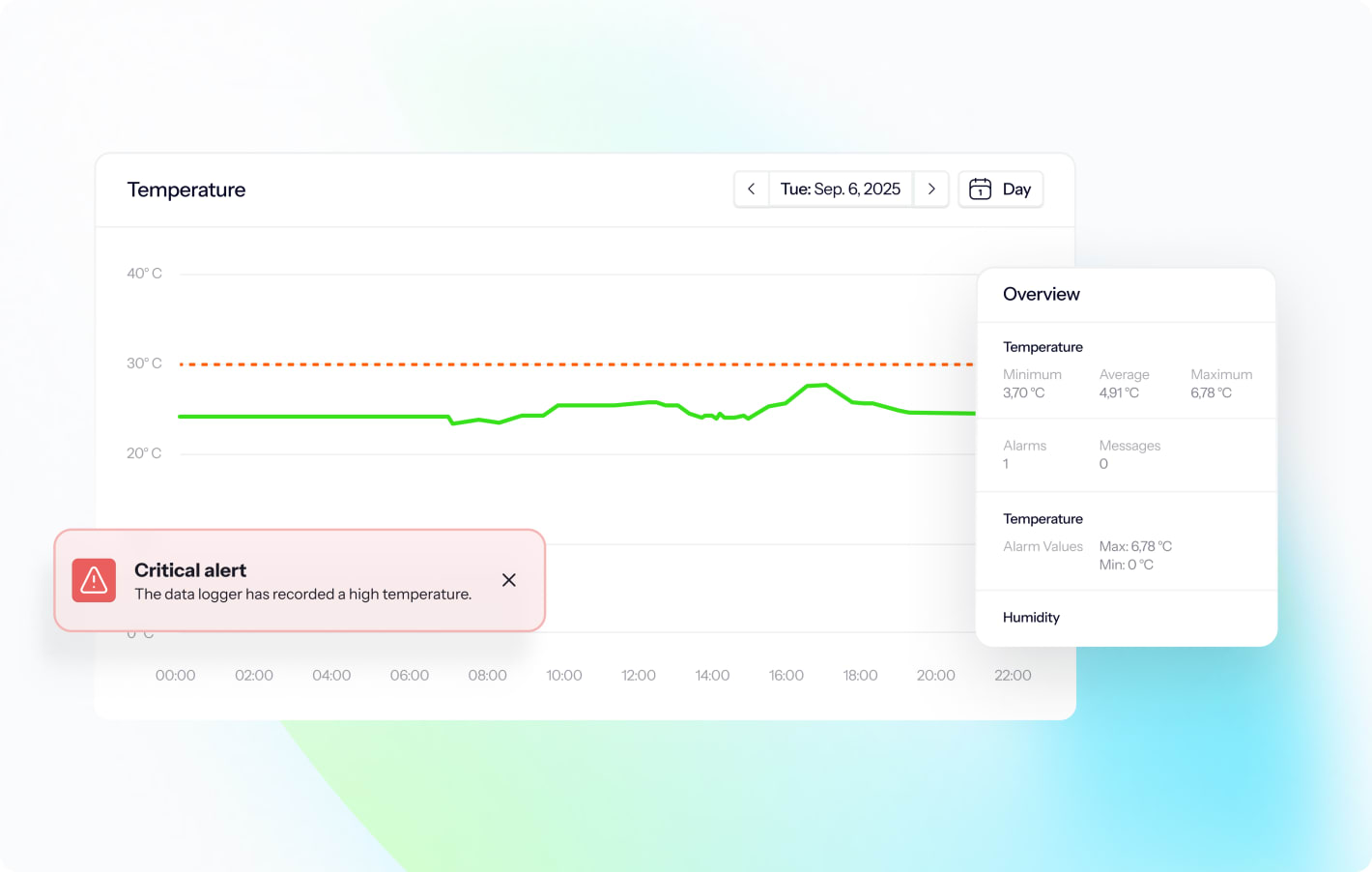
Real-time alerts notify teams immediately when temperatures exceed defined limits. Multi-channel notifications via email, SMS, and push notifications ensure critical alerts reach the right people regardless of their location or time of day.
Effective alert strategies balance sensitivity with operational reality. Risk-based approaches align alarm limits with product sensitivity, storage duration, and MKT calculations defined in USP 1079.2. This reduces alert fatigue while ensuring critical events receive attention.
Also read: How to investigate a temperature excursion faster
Where is cold chain monitoring used in pharmaceuticals?
Manufacturing and laboratory storage
Pharmaceutical manufacturers monitor ingredient storage rooms, in-process areas during formulation, stability chambers for product testing, and finished product warehouses. Facility monitoring with permanent sensor networks provides continuous qualification, while shipment monitoring tracks materials during inbound and outbound logistics.
Distribution and logistics
Cold chain distribution represents the highest-risk phase for temperature-sensitive products. Distribution monitoring strategies depend on product risk and distribution complexity. High-value biologics may require continuous monitoring with real-time GPS tracking throughout transport. More stable products might use periodic monitoring at distribution centers with validation data demonstrating transport containers maintain specification.
Healthcare facilities and pharmacies
Healthcare facilities and pharmacies represent the final storage phase before patient administration. Monitoring systems for healthcare settings should prioritize simplicity and reliability over advanced features. Plug-and-play deployment, automated calibration reminders, and straightforward reporting help ensure compliance even with minimal technical support.
Also read: Trend in cold chain monitoring: Moving to facility control

What are the key components of a cold chain monitoring system?
Wireless connectivity and cloud storage
Modern pharmaceutical monitoring systems use WiFi or cellular networks to transmit data from sensors to cloud platforms. Network redundancy prevents data loss during connectivity interruptions. Systems buffer readings locally when WiFi drops, then automatically sync when connections restore.
Cloud-based storage provides secure, scalable data retention that meets regulatory requirements for electronic records. Platforms must support 21 CFR Part 11 compliance with audit trails, electronic signatures, and role-based access controls. Historical data remains accessible for the required 5-year minimum retention period.
Calibration and accuracy assurance
All monitoring equipment requires regular calibration to maintain measurement accuracy. ISO 17025 accredited calibration ensures sensors meet pharmaceutical-grade accuracy requirements and remain traceable to international standards like NIST or PTB.
Annual calibration is standard for most pharmaceutical applications. On-wall calibration methods reduce downtime by eliminating the need to remove sensors from service during the calibration process. Automated systems track calibration due dates and generate alerts before certificates expire, preventing lapses in compliance.
Validation and qualification
Temperature monitoring systems require validation to demonstrate they perform as intended under all expected conditions. Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols document system configuration, functional testing, and real-world performance.
Validation documentation must demonstrate the system meets user requirements, operates reliably across defined conditions, and integrates properly with existing quality management systems. Change control procedures govern modifications to hardware, software, or operational parameters.
Monitoring system selection checklist
For cold chain monitoring
Evaluate cold chain monitoring systems against 70 GxP compliance requirements. Compare capabilities and identify gaps before making your decision.
Get instant access to a structured framework to assess cold chain monitoring systems.

What are the GxP compliance requirements for cold chain monitoring?
EU GDP and monitoring obligations
EU Good Distribution Practice Guidelines establish comprehensive requirements for temperature monitoring throughout pharmaceutical distribution and mandates continuous or periodic monitoring with alarm systems that enable rapid intervention when deviations occur.
Key requirements include documented procedures for monitoring, calibrated equipment with valid certificates, defined alarm limits based on product requirements, and immediate investigation protocols when excursions occur. The requirements also cover risk-based approaches that align monitoring intensity with product sensitivity and distribution complexity.
Also see: GDP requirements and guidelines for pharma
USP 1079 series and storage guidelines
The USP 1079 series provides comprehensive guidance on storage and distribution practices for finished drug products. These chapters replaced earlier USP 1118 guidance and now include specific sub-chapters addressing monitoring devices, temperature mapping, and Mean Kinetic Temperature calculations.
USP 1079 emphasizes risk-based monitoring strategies that account for product stability profiles, storage duration, and distribution pathways. USP 1079.2 specifically addresses Mean Kinetic Temperature, providing scientifically defensible methods to evaluate cumulative temperature exposure during excursions.
Also see: Selection guide: Cold chain monitoring devices and sensors
WHO TRS 961 and transport requirements
WHO Technical Report Series 961, Annex 9, establishes international standards for time and temperature-sensitive pharmaceutical products. The guidance covers both storage and transport, with particular focus on cold chain management for vaccines and biologics requiring strict temperature control.
WHO guidance mandates continuous temperature monitoring for high-value and temperature-critical products during transport. Organizations must demonstrate through thermal validation that their distribution methods maintain products within specification under worst-case conditions including seasonal extremes and transport delays.
Also see: What are the WHO’s guidelines for temperature mapping?
21 CFR Part 11 and electronic records
FDA 21 CFR Part 11 establishes requirements for electronic records and electronic signatures used in pharmaceutical manufacturing and distribution. Any monitoring system that replaces paper documentation must comply with Part 11 controls for data integrity, access management, and audit trails.
Core Part 11 requirements include secure user authentication, tamper-proof audit trails documenting all system access and data modifications, electronic signature capabilities, and validation demonstrating the system prevents unauthorized access or data manipulation.
How do you select a cold chain monitoring system?
Effective system selection begins with clear requirements that reflect your operational needs, regulatory obligations, and quality management goals. Core requirement categories include measurement specifications like temperature ranges and accuracy requirements, connectivity needs, compliance features including 21 CFR Part 11 support, scalability factors, and integration requirements with existing systems.
Not all monitoring vendors provide equivalent capabilities for pharmaceutical applications. Evaluation should focus on regulatory experience, technical competence, and long-term support capacity. Critical criteria include whether the vendor provides GxP-compliant systems with validation support, offers ISO 17025 accredited calibration, demonstrates pharmaceutical industry experience, provides comprehensive technical support, and maintains systems through ongoing software updates.
Purchase price represents only one component of monitoring system costs. Total cost of ownership includes hardware and software costs, installation and commissioning, calibration and maintenance, training, validation activities, and ongoing support fees. Continuous monitoring systems often show lower total cost of ownership than periodic approaches despite higher initial investment.
FAQ about pharmaceutical cold chain monitoring

Monitor your entire cold chain in ONE solution
Get complete cold chain visibility in one platform – with real-time alerts, automatic documentation, and unified compliance across your entire network. See how it works in the catalog.
One solution, control over your entire cold chain
– from warehouse to delivery
- Automated alerts and documentation designed for GDP
- One platform for your entire cold chain – in real-time
- Monitor temperature, GPS, shock, humidity, and light

