How to select cold chain monitoring devices and sensors
for pharmaceutical compliance

Adam Hartmann-Kruckow
Everything you need to compare cold chain monitoring devices and sensors for pharma – from USB data loggers to wireless sensors and real-time IoT systems – based on cost, compliance requirements, and operational fit for pharmaceutical distribution.
Get a structured framework to assess vendors for GxP cold chain monitoring systems.
Intro
Pharmaceutical cold chain monitoring depends on selecting devices that deliver GxP-compliant data with the accuracy, connectivity, and validation support your operation requires.
This guide compares sensor types, data logger technologies, and monitoring architectures to help you choose equipment that meets regulatory requirements while fitting your operational workflow.
The wrong monitoring devices create compliance gaps, generate unreliable data, and require expensive replacements during validation. Understanding device capabilities before purchase prevents costly mistakes and ensures your monitoring system performs reliably throughout its lifecycle.
Table of contents
- What types of cold chain monitoring devices exist?
- How do you choose between device types?
- What sensor types are used for cold chain monitoring?
- What accuracy and calibration standards apply?
- 4 considerations when evaluating cold chain monitoring devices
- What connectivity options work for cold chain monitoring?

Quick definitions
- Sensors: The physical component that measures conditions.
- Data loggers: Devices with integrated (internal or external) sensors that record and store data internally.
- Monitoring systems: Connected platforms that data loggers transmit data to.

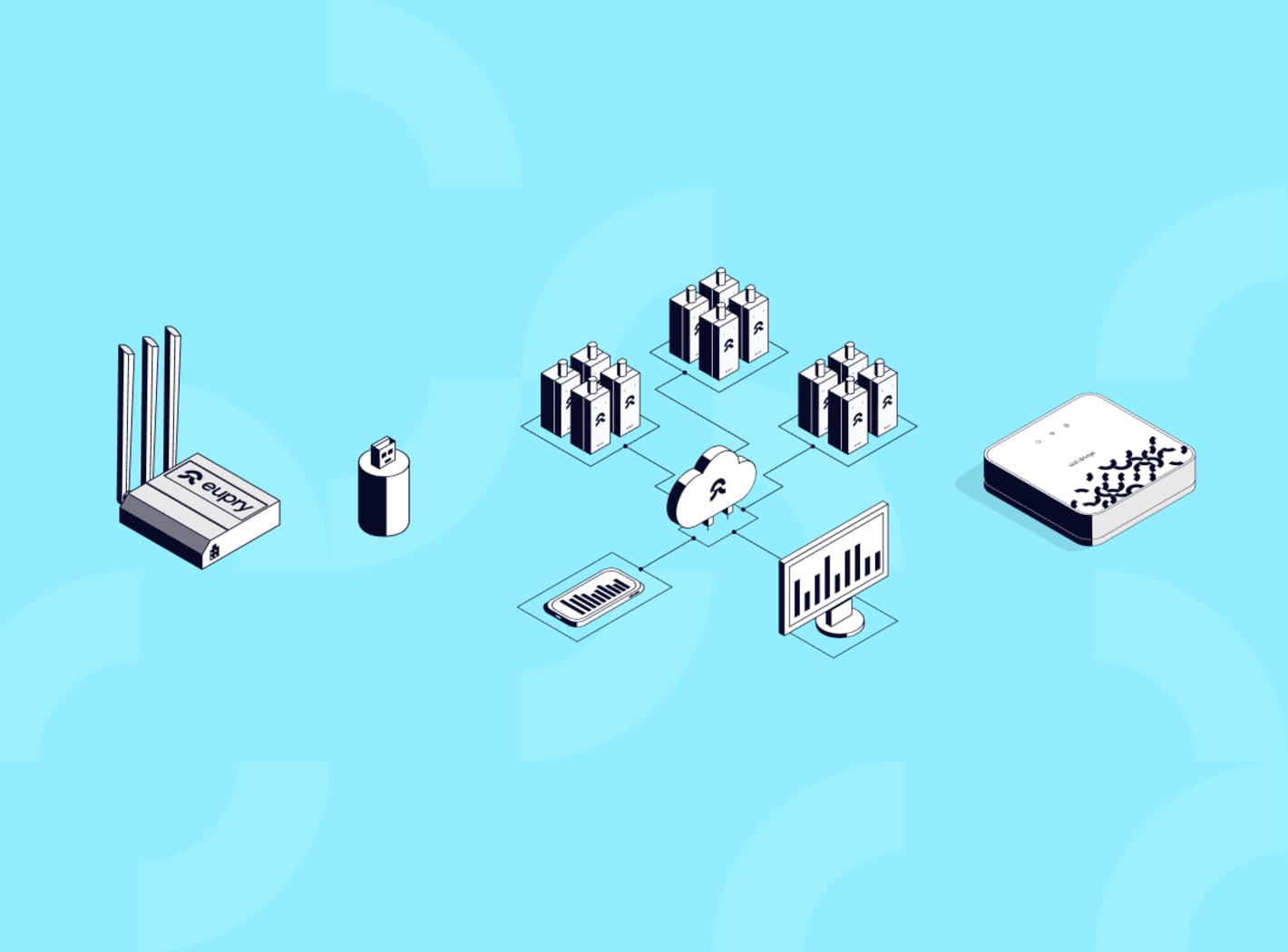
What types of cold chain monitoring devices are available?
Pharmaceutical cold chain monitoring relies on several device categories, each designed for different storage environments and operational requirements.
- USB data loggers are self-contained devices that record temperature data on internal memory. Staff retrieve data by connecting the logger to a computer via USB cable. USB loggers work well for shipment monitoring, short-term validation studies, or facilities without network infrastructure. They are battery-powered, portable, and require no IT integration. Cost: $50-400 per device depending on accuracy and temperature range.
- Wireless data loggers measure and transmit temperature data automatically to cloud-based platforms via Wi-Fi or cellular networks. They eliminate manual data collection and provide real-time visibility into storage conditions across refrigerators, freezers, warehouses, and distribution centers. Wireless loggers enable immediate response to temperature excursions and centralize compliance documentation for multi-site operations. Cost: $200-600 per device plus software subscription.
- Wired sensor systems connect multiple temperature probes to central data acquisition units through physical cables. These systems work well in large cold storage facilities, warehouse installations, or transport containers where permanent infrastructure and high sensor density are required. Wired systems provide reliable data transmission without depending on wireless networks. Cost varies significantly based on sensor count and installation complexity.
- Transport-specific devices include single-use temperature indicators for shipments, reusable shipping loggers with impact and GPS tracking, and container monitoring systems for pharmaceutical distribution. These devices must withstand vibration, handle extreme temperature ranges during transport, and provide tamper-evident documentation for chain-of-custody requirements.
Your device selection depends on whether you need real-time alerts, how many points are required, existing infrastructure, and whether you are monitoring fixed storage or transit.
Also see: Guide for temperature monitoring in GxP
What is the difference between sensors, data loggers, and monitoring systems?
Understanding these terms prevents confusion when evaluating cold chain monitoring solutions.
Sensors are the physical measuring components that detect temperature changes. The three sensor types used in pharmaceutical applications - thermocouples, RTDs, and thermistors - convert temperature into electrical signals. Sensors alone cannot record, store, or transmit data. They require connection to data logging equipment.
Data loggers are complete recording devices that combine sensors with memory, processors, battery power, and data transmission capabilities. A data logger captures sensor measurements at defined intervals (every 1-15 minutes typically), stores thousands of readings in internal memory, and transfers data either manually via USB or automatically through wireless connectivity. When buyers say "I need monitoring devices," they typically mean data loggers with integrated sensors.
Monitoring systems are the complete solution architecture that connects multiple data loggers to centralized software platforms. The system includes the physical loggers, cloud-based software for data visualization and alerting, mobile access for remote viewing, automated reporting for audits, and documentation management for compliance. Monitoring systems provide the infrastructure for managing cold chain compliance across entire facilities or distribution networks.
Practical distinction: You purchase data loggers (hardware) as part of a monitoring system (complete solution). Some advanced systems use replaceable sensor tips that attach to permanent logger housings - this approach reduces calibration time and cost since you swap sensors rather than entire devices.
How do you choose between cold chain monitoring device types?
Choose USB data loggers when:
- Monitoring individual shipments where cost per unit matters
- Conducting short-term validation studies (1-7 days)
- Network connectivity is unavailable or prohibited
- Manual data review after completion is acceptable
- Lowest upfront device cost is essential
Choose wireless monitoring systems when:
- Monitoring refrigerators, freezers, warehouses, or cold rooms continuously
- Managing temperature compliance across multiple storage locations
- Immediate alerts for excursions are operationally necessary
- Centralized audit documentation across sites is required
- Eliminating manual data downloads reduces compliance risk
Choose cellular-connected devices when:
- Tracking shipments requiring real-time visibility during transport
- Monitoring remote locations without facility Wi-Fi infrastructure
- GPS tracking and route documentation are needed alongside temperature
- Customers require live shipment data access during transit
- Operating across multiple carriers or transportation modes
Your monitoring environment (storage vs. transport), infrastructure availability, and regulatory documentation requirements determine which devices fit your operation.
What sensor types are used for pharmaceutical cold chain monitoring?
Pharmaceutical monitoring devices use three core sensor technologies, each suited to different applications.
- Thermocouples (Type K most common) offer wide temperature ranges (-200°C to +350°C) and fast response times, making them the standard for temperature mapping studies. They require external data loggers and regular calibration.
- RTDs deliver superior accuracy (±0.1°C to ±0.3°C) and long-term stability, making them the preferred choice for critical applications like vaccine storage and biologics manufacturing where precise control is essential. Platinum RTDs (Pt100, Pt1000) cost more but require less frequent calibration.
- Thermistors provide excellent sensitivity in narrower ranges (-50°C to +150°C) and are commonly integrated into standalone data loggers. However, they're less stable than RTDs over time and need more frequent calibration verification.
Your sensor choice affects measurement accuracy, calibration frequency, and total cost of ownership.
Also read: ISO 17025 accredited calibration explained
How do data loggers differ from monitoring systems?
The distinction between standalone data loggers and integrated monitoring systems affects both operational workflow and compliance capabilities.
Standalone data loggers are self-contained devices with integrated sensors, memory, and battery power. They record temperature internally, requiring manual download via USB or proprietary software. Loggers work well for transport monitoring, short-term studies, or facilities with limited IT infrastructure. The tradeoff: manual data collection, risk of data loss between downloads, and no real-time visibility into storage conditions.
Integrated monitoring systems connect multiple sensors to centralized platforms through wired or wireless networks, transmitting data continuously to cloud-based software. Remote temperature monitoring provides real-time dashboards, automated alerts, and on-demand reporting across multiple sites. Higher initial investment, lower ongoing operational costs through reduced labor and faster excursion response.
Hybrid approaches use wireless data loggers transmitting to local gateways, balancing real-time visibility with battery-powered flexibility for complex facilities or areas with limited network coverage.


Get a cold chain monitoring system selection checklist
Get a structured framework to assess vendors for cold chain monitoring systems in GxP.
What accuracy and calibration standards apply to monitoring devices?
Device specifications determine whether your monitoring data satisfies regulatory requirements and supports quality decisions.
Measurement accuracy for pharmaceutical applications typically requires ±0.5°C or better across the operating range per WHO guidelines. High-value products like biologics and vaccines often demand ±0.2°C to ±0.3°C for narrow validated ranges. Device specifications should state accuracy as maximum deviation across the full operating range, not just at specific calibration points.
Calibration frequency follows WHO Technical Report Series 961 recommendation of annual calibration as minimum standard. All calibration must trace to ISO 17025 accredited standards with documented traceability to national reference standards (NIST, PTB). Calibration certificates must document actual measured values at multiple temperature points, not just "pass" statements.
Modern on-wall calibration methods significantly reduce downtime compared to traditional calibration.
4 components to consider
How to evaluate cold chain monitoring devices in GxP
Device evaluation requires assessing regulatory, technical, and operational capabilities.
- Regulatory compliance: Systems must support 21 CFR Part 11 requirements including audit trails, electronic signatures, role-based access controls, and data integrity safeguards. Devices should include validation support packages with IQ/OQ/PQ protocols and vendor documentation demonstrating regulatory compliance.
- Technical specifications: Compare temperature range coverage, measurement accuracy, response time, data collection intervals, local storage capacity during outages, and battery life for wireless devices. Verify specifications reflect real-world performance, not just laboratory conditions.
- Operational fit: Evaluate installation complexity, calibration logistics, software usability, alert configuration, reporting capabilities, and technical support. Devices meeting technical specifications but creating operational burden often fail to deliver expected benefits.
- Total cost of ownership: Include purchase price, installation, calibration, validation, software subscriptions, training, and ongoing support. Continuous monitoring systems often show lower five-year costs despite higher initial investment through eliminated manual labor, reduced re-mapping frequency, and prevented product losses.
Also read: Thermal validation guide: Planning, execution, and documentation
Compare devices systematically
Download our evaluation framework with technical specifications, compliance requirements, and TCO calculations.

What connectivity options work for cold chain monitoring?
Your connectivity choice affects data reliability, IT integration complexity, and validation requirements.
Wi-Fi connectivity integrates with existing IT infrastructure and provides high bandwidth for data transmission. Pharmaceutical systems typically connect to dedicated networks separate from corporate systems for security and validation simplicity. Wi-Fi requires reliable coverage across all locations, which can challenge older facilities with concrete walls or metal equipment.
Cellular connectivity eliminates IT infrastructure dependencies by transmitting directly to cloud platforms through cellular networks. This works well for remote locations, temporary installations, or organizations avoiding IT dependencies during deployment. Requires ongoing data plans and adequate cellular coverage.
Proprietary wireless protocols (sub-GHz radio, mesh networks) offer alternatives when WiFi coverage is problematic. These create dedicated wireless networks using gateways that collect and relay sensor data. Better wall penetration than WiFi, but require additional infrastructure and may complicate IT security assessments.
All quality systems buffer data locally during network interruptions, automatically syncing when connectivity restores to prevent data loss.
Monitoring system selection checklist
For cold chain monitoring
Evaluate cold chain monitoring systems against 70 GxP compliance requirements. Compare capabilities and identify gaps before making your decision.
Get instant access to a structured framework to assess cold chain monitoring systems.

FAQ about cold chain monitoring devices
Monitor your entire cold chain in ONE solution
– from warehouse to delivery
- Automated alerts and documentation designed for GDP
- One platform for your entire cold chain – in real-time
- Monitor temperature, GPS, shock, humidity, and light

Monitor your entire cold chain in ONE solution
Get complete cold chain visibility in one platform – with real-time alerts, automatic documentation, and unified compliance across your entire network. See how it works in the catalog.

