Temperature compliance for healthcare logistics
Reduce risks, manual work, and costs with reliable, harmonized temperature monitoring, mapping, and calibration designed for the diverse needs of healthcare logistics.
- GDP compliance in weeks, not months
- Audit reports in 3 clicks
- Standardized processes across sites
- 20-30% reduced costs


You can't win pharma without proving GDP compliance
... but getting there and that is both complex and time-consuming.
Dozens of vendors (and problems)
Validation consultants. Monitoring systems. Calibration services. Each with different processes, timelines, and bills. Managing multiple vendors is complex and costly.
Validations delay expansion and growth
Every warehouse layout change means re-mapping. Every new facility sits idle waiting for final validation. Your expansion plans wait on other's schedules.
Quality teams spread too thin
QA firefight across sites instead of building systems. Local teams are disturbed. Local problems become global headaches. Compliance is reactive, not strategic.
Costs that spiral without warning
Surprise calibration costs. Emergency re-mapping fees. Last-minute consultant rates. Growth is expensive when every expansion restarts the cycle and vendors are scattered.
- The opportunity: Healthcare logistics is the most lucrative segment in cold chain.
- The challenge: You only get those deals if you prove GDP compliance (fast).
Eupry is build to solve this challenge.
Why choose Eupry in healthcare logistics?
Pharma clients are among the top most valuable segments for logistics, but they also come with the strictest standards.
Our system was built for this complexity.
We provide the expertise and services to create a solid foundation on which healthcare logistics providers can expand their pharma operation - faster, cheaper, and with no operational downtime.
- Beat competition
- Prove GDP compliance
- Minimize vendors (all you need is 1)
- Reduce costs by 20-30%

Trusted by 500+ pharma and healthcare logistics leaders
What makes Eupry the right fit for pharma logistics?
Healthcare logistics providers use Eupry's services to prove GDP compliance quickly, cut vendors, and reduce costs.
- Validation expertise that gets logistics: Our team has qualified hundreds of pharma facilities and know what is required.
- Technology designed for multi-site networks: One dashboard for all sites, standard global processes, and fast, scalable deployment.
- Integrated platform = no vendor chaos: Instead of juggling vendors for mapping, monitoring, and calibration, you get one partner who handles everything.
- Speed that wins contracts: GDP-ready in months means you can bid on contracts while competitors are still validating their first site.
- No more re-mapping: Our continuous mapping solution eliminates disruptive re-mappings. Maintain compliance without shutting down your facilities.
Monitoring, continuous mapping, and calibration in ONE platform
Bring temperature and humidity compliance – monitoring, calibration, and mapping – into one digital, GxP-compliant solution, providing one centralized overview of all data and one process.
- Reduce total cost of ownership
- Eliminate waste of time spent on manual tasks
- Gain full control (and GDP-compliance) across facilities

With Eupry, you get
CEIV & GDP ready
The solutuon is built for GDP and CEIV, and both mapping and monitoring requirements satisfied.
20-30% lower costs
20-30% lower total cost of ownership over 3-5 years. Automate and go from 10, 20, 30, or 100+ vendors to 1.
Higher quality levels
2-6x richer data for better decisions, predictive mainentance, and proactive quality management.
Central control of all sites
Same SOPs, same dashboards, same documentation for all facilities and unit across your network.

Temperature mapping services: Fast validation + continuous compliance
Get your facilities GDP-ready in weeks.
Engineer-led temperature mapping validates your warehouses or other storage facilities quickly, and then continuous mapping eliminates periodic re-mappings.
- Fast GDP validation: Fast project delivery and flexible solutions.
- Digital mapping: Digital test plans and reporting from anywhere.
- Lower costs: 20-30% lower total cost of ownership.
- Higher quality: Well-tested processes, audit support, and more.

Live automated monitoring = central control across all sites
Wi-Fi-based monitoring provides one centralized view of temperature data across your entire logistics network.
- See every facility on one dashboard
- CEIV and GDP ready by design
- Audit reports in 3 clicks
- Proactive alerts via email/SMS

Accredited on-the-wall calibration: 95% faster, zero downtime
For a large logistics facility, traditional calibration might take days; Eupry's ISO 17025-accredited on-the-wall calibration takes under an hour.
Swap sensor tips in seconds without removing loggers, and reduce calibration time by up to 95%.
No device removal, no downtime, no data gaps.
Get an instant overview of how the solutions work.


Download a free product catalog
See all the technical details and learn how the GDP-ready temperature compliance solutions work.
Legal basis and guidance
The solutions are designed for GDP with a legal basis in cGMP, USP <1079>, and EudraLex Volume 4 Part 1 Chapter 3, and based on guidance from WHO guidelines (supplement 8 and 7 and annex 8), DKD-R 5, FD X 15-140 (French standard), and the ISPE Standard “Controlled Temperature Chamber Mapping and Monitoring”.
CEIV Pharma ready in months, not years
Eupry's solution is designed for GDP and supports all requirements for CEIV Pharma certification.
- CEIV documentation: The platform provides all the temperature documentation that CEIV auditors expect.
- Faster certification: Logistics operators using Eupry can typically get CEIV Pharma certified in 6 months compared to the normal 12-18 months.
- All the support you need: Our team can help prepare documentation and support you through the certification process.
Get in touch with one of our GDP specialists for a no-strings-attached consultation.

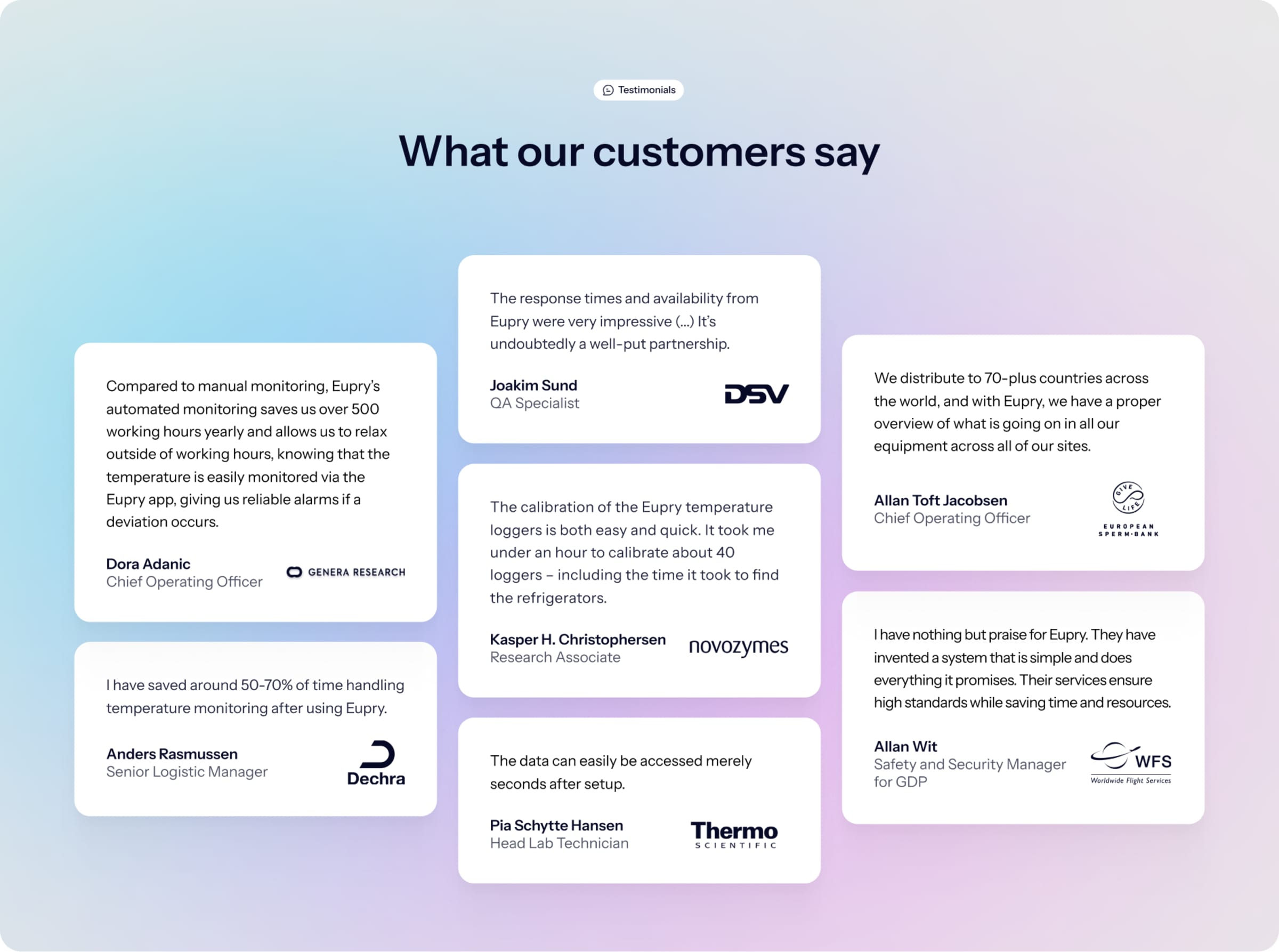
How DSV validated their GDP-compliant logistics center with Eupry
Before DSV Sweden could utilize their new logistics center, they needed to ensure its ability to maintain temperature conditions in a way that was both swift and GDP compliant.
The response times and availability from Eupry were very impressive. We have continuously been in contact with each other every step of the way. They helped us carry out various tests and assisted in the interpretation of the data – coming up with suggestions or feedback on whether the results were compatible with our requirements. It was undoubtedly a well-put partnership.
Joakim Sund, Quality Assurance Specialist at DSV

Don't take our word for it
Great service! The Eupry Team listens and provides a rapid response. That, added to their top-notch technology, provides accurate mapping/monitoring within industry rules and regulations and peace of mind.
Yolanda Rodriguez, Executive Operations Administrator, FedEx
We have saved around 50-70% of the time we spend on temperature monitoring.
Anders Rasmussen, Senior Logistic Manager, Dechra Pharmaceuticals PLC
Tailored for healthcare logistics
The service provides you with dependable, cost-effective, real-time temperature and humidity validation and monitoring tailored to the diverse needs of the pharmaceutical logistics industry.
The ISO-accredited solution is tailored to meet the requirements for logistics operations handling highly-regulated pharmaceutical and other sensitive products.

Client Testimonials
Built for GDP and GMP
All your compliance boxes? Consider them ticked.
- ISO 9001 certified
- ISO 17025 accredited
- ISO 27001 accredited
- 21 CFR Part 11 module
And much more.

High compliance rating by Novo Nordisk
Eupry Aps provided (…) documentation to conclude that procedures and efficient controls to ensure compliance are in place and well-functioning as intended in all key areas/processes (…)
ISO AUDIT REPORT by Novo Nordisk, June 2023

What is GDP compliance for logistics
GDP is the quality standard for distributing medicinal products. For healthcare logistics providers, GDP compliance means demonstrating that temperature-sensitive pharmaceuticals remain within specification throughout storage and transport.
Core GDP requirements for temperature compliance:
- GDP guidelines require qualified storage areas, which means performing temperature mapping to identify hot and cold spots.
- You need continuous temperature monitoring with automated alarms to catch deviations before products are affected.
- Calibration must be traceable and documented, typically requiring ISO 17025 accreditation.
The EU GDP guidelines (2013/C 343/01) and WHO Technical Report Series 961 set the framework. In the US, USP chapters <1079.2> and <1079.4> provide additional guidance on excursion evaluation and mapping frequency.
Why this matters for logistics
Pharmaceutical logistics is one of the highest-value segments in cold chain. But pharma clients only work with GDP-ready providers. CEIV Pharma certification - the industry gold standard - requires documented temperature compliance across your entire network.
Getting GDP-ready traditionally takes 12-18 months per facility. Continuous mapping and automated monitoring cut this to 6 months or less.
Also read: GDP requirements and guidelines











