IQ, OQ, PQ for incubators

Adam Hartmann-Kruckow
How to qualify incubators in pharma
Step-by-step guide for equipment qualification for incubators in pharma from IQ, OQ, and PQ. From URS and probe placement to recovery, alarms, and contamination controls.
Get templates aligned with EU GMP Annex 15 expectations and FDA terminology to speed up planning, execution, and documentation.

Note:
This guide is provided for general educational purposes only and does not replace official regulatory requirements or professional judgment. Users are responsible for ensuring that IQ, OQ, and PQ activities are performed in accordance with applicable regulations, including EU GMP Annex 15, FDA 21 CFR Part 211, and other relevant GxP standards. Protocol templates offered here are examples and must be adapted, reviewed, and approved within your company’s quality management system before use.
Incubators used in pharmaceutical development, QC, and microbiology must demonstrate stable temperature, CO₂, and often humidity under routine operation. Regulators expect evidence that the unit is installed as designed, operates as intended, and performs with representative loads.
Incubator qualification (IQ/OQ/PQ) is the formal validation process that proves your equipment was installed correctly, operates within specified limits, and performs reliably under actual use conditions. It's a regulatory requirement for any incubator used in GMP environments – from microbiology labs to stability chambers.
But qualification is just one piece of incubator compliance. Complete compliance also requires continuous temperature monitoring, periodic mapping studies, and regular calibration. If yo a're looking for a comprehensive guide covering all aspects of incubator compliance, see our [complete incubators guide](/applications/incubator-compliance /).
This page focuses specifically on the qualification process: What IQ, OQ, and PQ involve, how to plan your qualification studies, and when requalification is required.
Also read: How to write a URS for pharmaceutical storage areas and TCUs
What are IQ, OQ, and PQ for incubators?
IQ (installation qualification), OQ (operational qualification), and PQ (performance qualification) are the core stages of equipment qualification. For incubators, especially CO₂ incubators, they prove correct installation, effective control of temperature/CO₂/humidity, and reliable performance in day-to-day culture work. Other incubators, such as stability or dry incubators, may also require qualification if they support regulated processes.
Also read: IQ, OQ, PQ in pharmaceuticals: Complete guide to equipment qualification and compliance
Why do incubators need IQ, OQ, PQ qualification?
Culture viability and contamination risk depend on stable environmental conditions and reliable alarms. Qualification provides documented assurance that the incubator maintains specification in realistic use – including door openings and recovery.
- GMP alignment: Risk-based qualification with traceability from URS to executed tests and results.
- Data integrity: Electronic records, audit trails, and access control for monitoring and mapping systems.
- Contamination control: Verification of cleaning/sterilization functions, HEPA filters, and materials of construction suitable for disinfectants.
Also read: Temperature monitoring for GMP and GDP compliance
How to plan IQ, OQ, PQ for incubators?
A good plan prevents false failures and rework. Focus on setpoint accuracy at 37 °C, CO₂ at 5 %, humidity targets, and realistic door-opening patterns.
- URS definition: Specify ranges and tolerances for temperature, CO₂, and humidity; define recovery and uniformity.
- Risk assessment: Identify failure modes – sensor drift, door-seal leaks, CO₂ supply issues, condensation, and contamination.
- Acceptance criteria: Make pass/fail binary – for example, uniformity ≤ 0.5 °C and CO₂ stability within ±0.5 %.
- Probe strategy: Plan mapping grid and reference instruments traceable to ISO/IEC 17025.
- SOP alignment: Include loading, cleaning, sterilization cycles, alarm response, and CO₂ cylinder changeover.
Also read: Temperature mapping guidelines for GxP

Download your free IQ/OQ/PQ protocol pack
Get instant access to an all-in-one protocol pack: From URS checklists to IQ, OQ, and PQ protocol templates. All you need to plan the qualification of temperature-controlled equipment and environments in pharmaceuticals, biotech, and logistics, aligned with WHO, FDA, GMP/GDP/GxP guidelines.
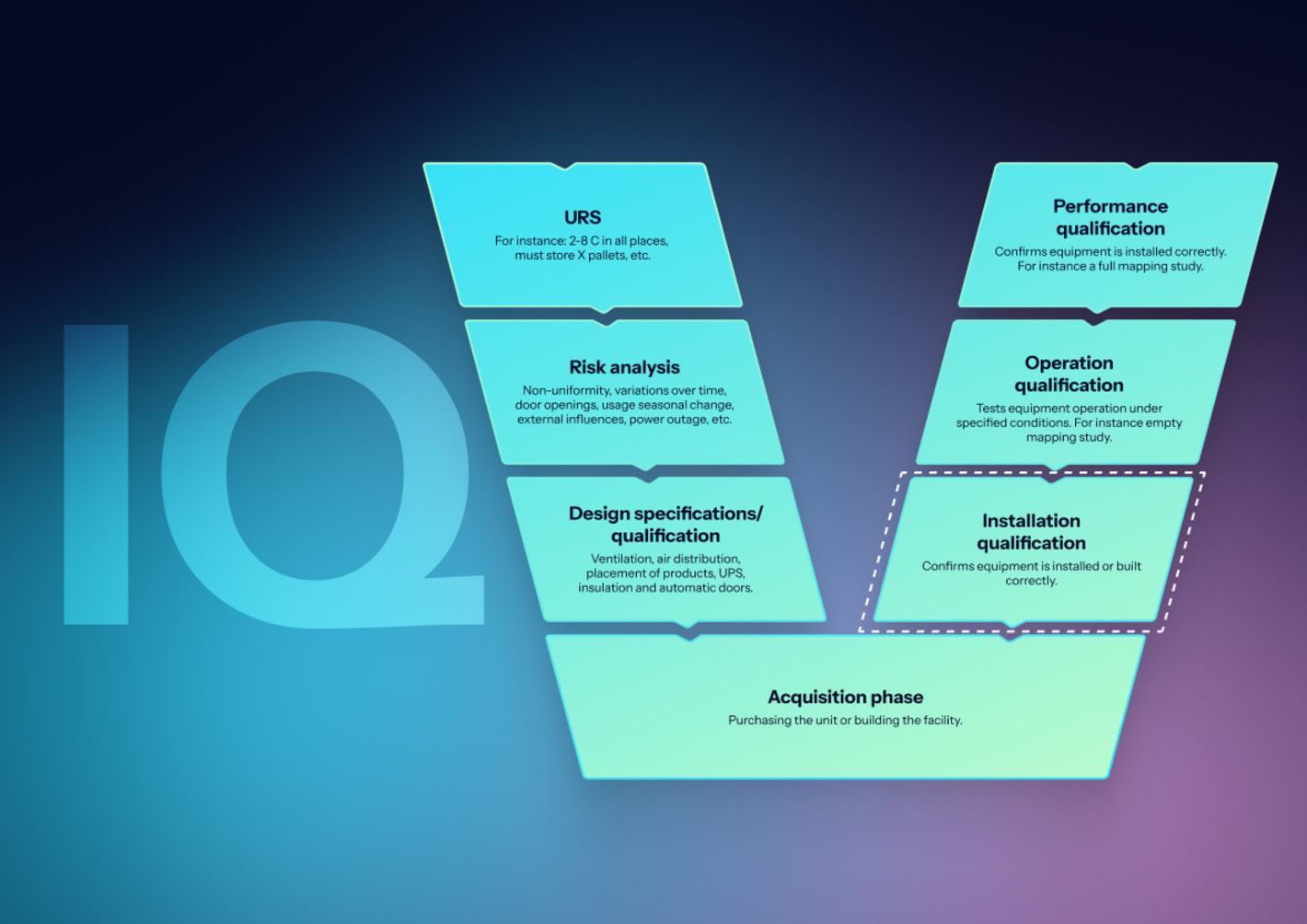
How to perform IQ for incubators?
IQ confirms the unit and utilities match the design and are ready for qualification.
- Equipment identification: Record model, serial number, firmware/software, chamber volume, and accessories.
- Utilities and installation: Verify electrical supply, earthing, CO₂ line integrity, regulators, leak checks, and CO₂ gas purity certificates.
- Calibration evidence: Confirm reference probes and onboard sensors have traceable calibration.
- Configuration baseline: Record setpoints, alarm limits, notification paths, and user roles.
- Documentation control: Manuals, wiring diagrams, CO₂ certificates, and filter specs under document control.
Acceptance criteria: Installation conforms to URS, safety, and manufacturer requirements.
Also read: How to create a temperature mapping protocol
How to perform OQ for incubators?
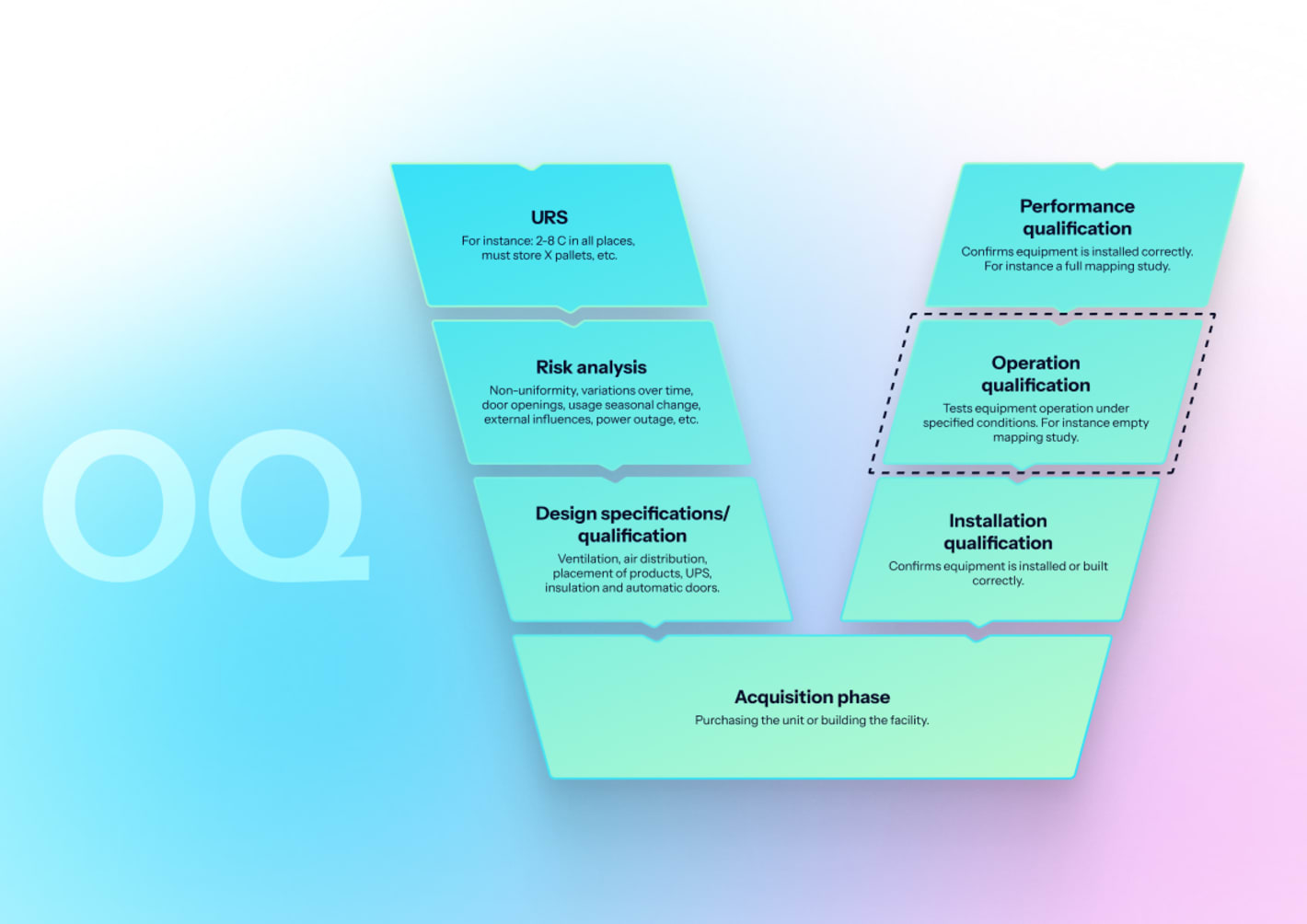
OQ challenges control and alarm functionality under test conditions, including door events, load simulants, and power interruption.
- Functional checks: Verify displays, controls, door seals, fan operation, and CO₂ delivery.
- Setpoint accuracy: Test temperature, CO₂, and (if used) humidity at setpoints; verify control stability.
- Uniformity and recovery: Map chamber grid; measure recovery after door openings and CO₂/temperature disturbances.
- Alarm challenges: Simulate high/low temperature, CO₂ out-of-range, door-left-open, and power loss; verify notifications locally and remotely.
- Data integrity checks: Confirm time synchronization, audit trail, user access control, and secure data export.
Acceptance criteria: Alarms behave as specified; uniformity and recovery meet limits; data integrity controls function.
Also see: Incubator compliance guide: monitoring, mapping, calibration, & qualification
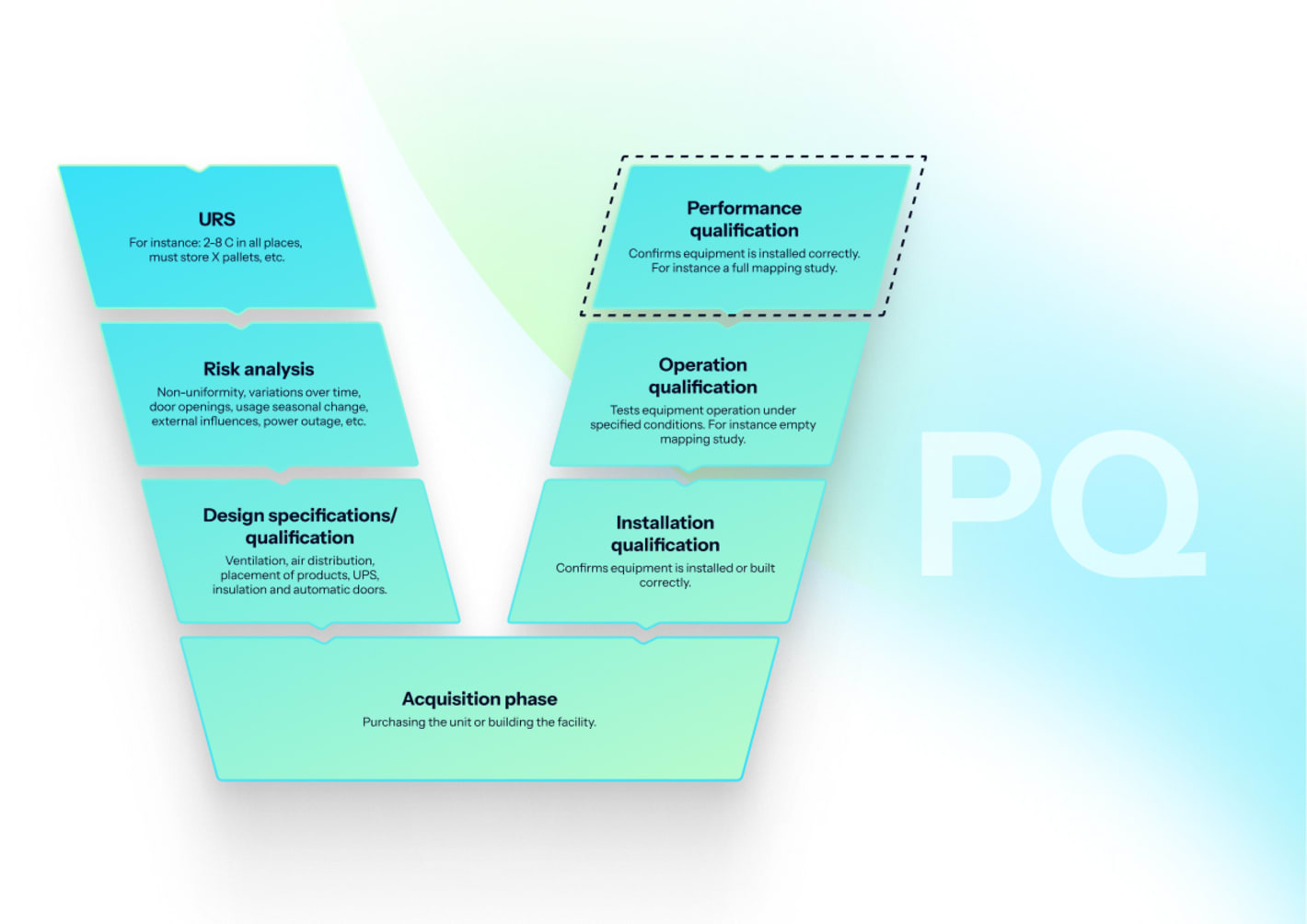
How to perform PQ for incubators?
PQ demonstrates performance in routine operation with representative loads and workflows.
- Representative loading: Use typical culture ware or simulants to represent thermal mass and airflow restrictions.
- Operational mapping: Confirm stability during routine door cycles and daily operations.
- Contamination control: Verify SOPs for cleaning, sterilization cycles, filter changes, and environmental monitoring where applicable.
- Process capability: Use monitoring data to document excursion rarity, duration, and appropriate response.
Acceptance criteria: Incubator maintains set ranges with routine use; alarms and procedures are effective.
Also read: Continuous temperature mapping explained
What documentation do auditors check during incubator qualification?
Auditors expect traceability from URS to executed tests, with clear mapping and alarm evidence. Missing calibration certificates and weak recovery tests are common findings.
- Traceability matrix: Links URS and risks to IQ/OQ/PQ tests and results.
- Mapping reports: Grid design, probe IDs, placement diagrams, raw data, and uniformity analysis.
- Calibration records: Reference instruments and onboard sensors with traceable certificates.
- Deviations and CAPA: Impact assessment, root cause, actions, and effectiveness checks.
- Change control: Repairs, controller updates, filter changes, and relocation records.
- Logbooks and training records: Evidence of correct use, maintenance, and staff qualification.
Also read: GxP glossary: Key definitions
How to reduce requalification workload for incubators?
Risk-based strategies and smarter data reduce repeated mapping cycles while strengthening compliance.
- Continuous mapping: Align fixed monitoring sensors with mapping hot/cold spots to gather ongoing evidence.
- Risk-based triggers: Re-qualify after significant changes, repeated deviations, or negative trend analysis. Document risk assessments to justify reduced mapping.
- Central oversight: Use dashboards to compare units and sites; act early on drift and alarms.
- Standardized templates: Reuse approved IQ/OQ/PQ protocols across labs to speed approvals.
How Eupry simplifies incubator qualification and thermal compliance
Handle the entire qualification process in one system - and go from mapping to monitoring in just a few clicks.
- Integrated tools: Run OQ and PQ studies using the same sensors that will provide continuous monitoring afterward. No need for separate equipment.
- A single source of truth: All your data is gathered in one place and automatically transferred. No manual work, no data gaps, full audit trails across mapping, monitoring, and calibration.
- On-the-wall calibration included: ISO 17025-accredited calibration happens directly at the sensor location. No downtime, no risks to compliance.
- Audit-ready reports in 3 clicks: Generate complete qualification reports with all test data, acceptance criteria comparisons, calibration certificates, and deviation records.
Qualification is easier when it is integrated with your ongoing monitoring and calibration. Eupry brings qualification, monitoring, and calibration into one platform:
IQ, OQ, and PQ services for GMP/GDP
One-stop for your entire validation process
(and all other thermal compliance needs) Get your incubator - and any other TCU or facility - qualification done faster with Eupry’s services built for GMP and GDP.
- One provider for the full validation
- Cost- and time-optimized processes
- Full GxP compliance confidence
Also read: Our continuous mapping solution

FAQ about IQ, OQ, PQ for incubators
Download free IQ/OQ/PQ protocol template pack
Get a step-by-step set of protocol templates and checklists to plan and document IQ, OQ, and PQ for incubators and other temperature-controlled equipment, aligned with common auditor expectations in GMP and GDP.

Continuous CO2 incubator monitoring
for GLP and GMP
- Real-time alerts and live monitoring overview
- ISO 17025-accredited calibration included
- CO2, temperature, and humidity in one
