GDP temperature mapping for healthcare logistics
Launch pharma-ready facilities faster with specialized, fast deployable mapping services and continuous mapping that eliminates re-mapping downtime.
- Fast GDP validation: Fast project delivery and flexible solutions.
- Digital mapping: Digital test plans and reporting from anywhere.
- Lower costs: 20-30% lower total cost of ownership.
- Higher quality: Well-tested processes, audit support, and more.
Get an instant overview of all service options, equipment specifications, and more.
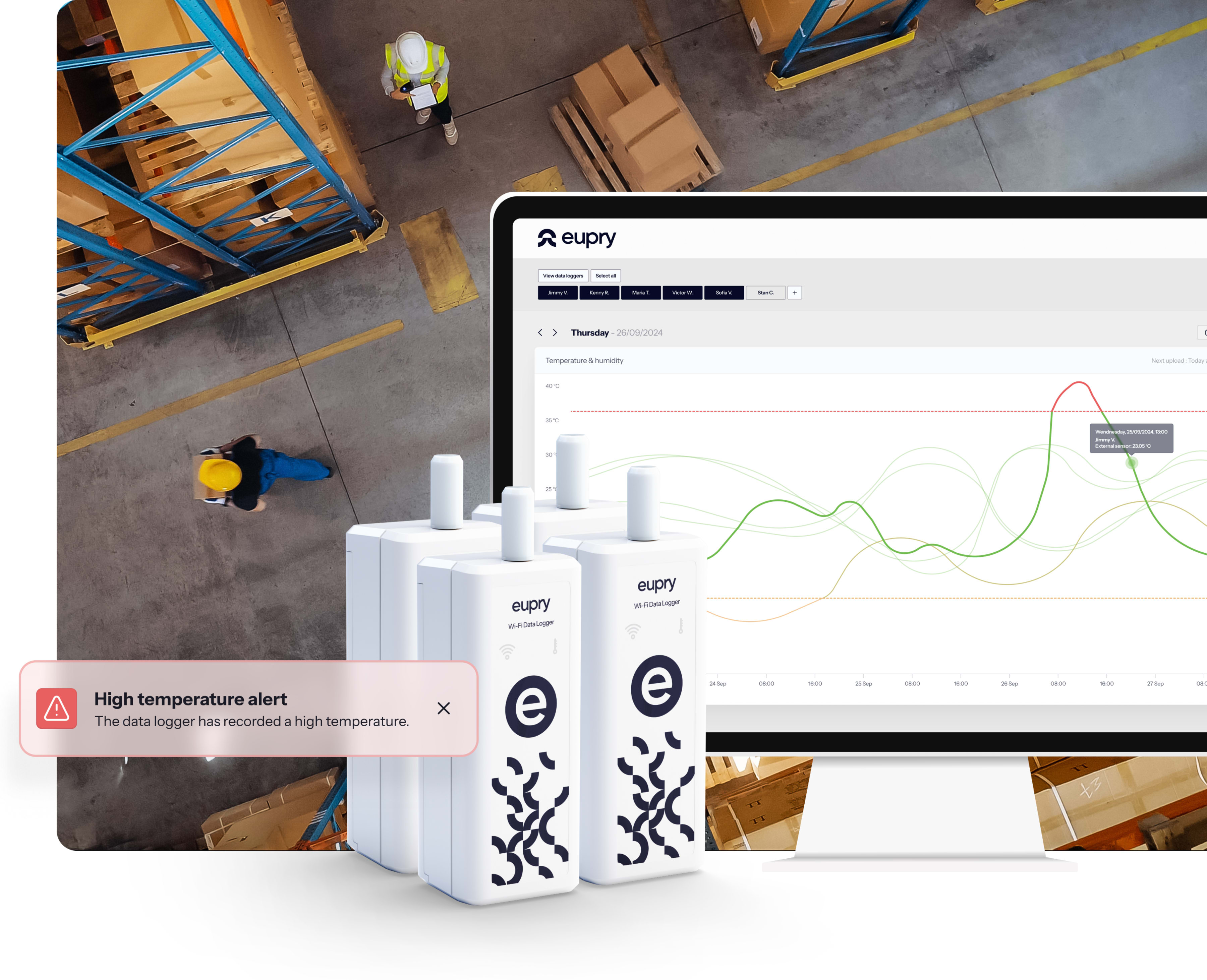
Trusted by +500 GxP-regulated companies worldwide
The mapping bottleneck of healthcare logistics
Manual errors = risks
Errors, lost data, and costly re-testing. Paper-based processes create risk and waste resources.
Operational disruption
Seasonal mappings, (urgent) re-mapping, and lengthy on-site work disrupts operations and shuts down facilities for days.
Inconsistent quality
Fragmented processes, multiple vendors, and disconnected systems create complexity, risks, costs, and red flags for pharma clients.
Complex requirements
Pharma is the top most valuable segments for logistics, but they also come with the strictest standards. GDP, USP <1079.4>, CEIV, etc. can be hard to translate into practice.
Lack of resources
New warehouses sit idle waiting for mapping studies. By the time consultants or internal teams are available, pharma contracts go to faster competitors.
Unpredictable costs
Emergency re-mapping fees. Calibration charges. Extra equipment costs. Last-minute consultant rates. Every facility expansion restarts the cycle.
Faster, GDP-tailored mapping services
(in less time, at lower cost, with full confidence)
Eupry delivers engineer-led GDP mapping in weeks, with continuous mapping that keeps facilities audit-ready without operational disruption.
With the fastest mapping solution you will find.
Digitalized global mappings
Roll the same process across sites in your network. Plan, execute, track, and document studies centrally. Create globally-approved test plans and export complete reports in minutes.
No operational disruptions
Choose on-site services with Eupry specialists or a guided remote option that keeps your operation moving. Loggers deploy quickly; calibration happens are done prior; and data is automatically transferred.
Made for pharma logistics
The solutuon is built for GDP and CEIV with tailored services and solutions for all healthcare logistics storage - from warehouse to cold rooms, and from ambient (15–25 °C), cold (2–8 °C), and below.
No more re-mapping
Make mapping a desktop exercise and transition to continuous temperature mapping. Eliminate costly, disruptive periodic re-mappings and maintain qualification without shutting down facilities.
20-30% lower costs
Get 20-30% lower total cost of ownership over a 3-5 year period with automation, no re-mapping costs, and going from 10, 20, 30, or 100+ vendors to 1.
Speed that wins pharma
Quickly deployable mapping solutions means no validation bottlenecks. Being GDP-ready in months means you can bid on contracts while competitors are still validating their first site.
How Eupry's mapping solutions work
Fast validation + continuous compliance
Get your initial mappings done in weeks, not months with engineer-led temperature mapping using digital tools built for GDP.
- Fast GDP validation: Fast project delivery and flexible solutions
- Digital mapping: Digital test plans and reporting from anywhere
- Higher quality: Well-tested processes, audit support, and more
- Pharma-level calibration: ISO 17025 calibrated equipment
- Audit support: Audit support and recommendations
See all the details of how the solutions work
Flexible options to fit your mapping
Pick a solution that fits your requirements (no more, no less) – from full on-site mapping services to rentable equipment. All options can be tailored to your needs and come with the same GxP-grade accuracy and compliance.
Choose between:
- Rentable mapping kits
- Remote mapping services
- On-site mapping services


Legal basis and guidance
The mapping solutions are designed with a legal basis in cGMP, USP <1079>, and EudraLex Volume 4 Part 1 Chapter 3, and based on guidance from WHO guidelines (supplement 8 and 7 and annex 8), DKD-R 5, FD X 15-140 (French standard), and the ISPE Standard “Controlled Temperature Chamber Mapping and Monitoring”.
Compliance and quality assurance
All your compliance boxes? Consider them ticked. Additionally, our specialists continuously update the service to adapt to evolving compliance standards, ensuring you remain fully compliant.
- GDP- and GMP-aligned methodology
- USP <1079.4> qualification expectations reflected in protocol and reporting
- CEIV considerations for logistics hubs (handover, alarm ownership, rapid audits)
- ISO 17025 accredited calibration & ISO 27001 certified information security
- FDA 21 CFR Part 11–ready electronic records and audit trails
- Optional IQ/OQ/PQ services for new or modified warehouses

Built for multi-site healthcare logistics
Launch facilities faster
Deploy mapping equipment in days. Complete studies in 1-2 weeks. Start accepting pharma products while competitors wait on consultants.
Standardize across sites
Same protocols, same documentation, same qualification standards everywhere. Pharma clients see operational maturity, not site-by-site variation.
Central quality oversight
Monitor validation status across all sites from one dashboard. Know which facilities are audit-ready, which need attention.
Support CEIV certification
Documentation ready for IATA CEIV Pharma and GDP audits. Both mapping and monitoring requirements satisfied.
Equipment built for GDP mapping in healthcare logistics
Whether you choose on-site mapping, a guided remote service, or a rentable mapping kit, you get the same GDP-compliant setup designed for pharmaceutical logistics. The result: Fast, accurate, and audit-ready data every time.
- Wireless data loggers, built for GDP mapping, sending results automatically over Wi-Fi so you can track your study without manual transfers or errors.
- ISO 17025 or traceable calibration, every logger arrives fully calibrated to GDP and CEIV standards, eliminating downtime and ensuring compliance.
- Validation software, manage your mapping digitally, review data in real time, and export GDP- and USP-ready reports instantly from anywhere.

Specialized in GDP and CEIV-level mappings
GDP, CEIV, and GMP compliance is what we do. After thousands of mappings, we understand the evolving standards of pharmaceutical compliance and can turn the complex requirements and operational needs of your healthcare facilties into audit-ready results.


Download a temperature mapping catalog
From on-site service to rentable mapping kits - see how all the mapping solutions work in the free catalog.
CONTINUOUS temperature mapping service
= Eliminate re-mapping
Instead of periodic snapshots, continuosu mapping gives you complete coverage verifying conditions daily, making mapping a desktop exercise instead of a week-long operational disruption.
- No shutting down for re-mapping
- Higher quality levels
- 20-30% lower costs
How it works
Traditional mapping uses temporary sensors for short studies. Continuous mapping uses a permanent monitoring setup to provide constant mapping-grade data.
Compliance: Follows risk-based validation principles consistent with WHO TRS 961, EU GDP guidelines, and FDA expectations.

How the on-site mapping service works
The ultimate solution if you need to meet requirements quickly. In this package, our team of validation professionals handles every step of the mapping process for you – planning, installation, and reporting.
- The project is scoped together with one of our specialists.
- Our validation team develops the protocol, and you review.
- Our specialists conduct the mapping study on your site.
- Your results are analyzed, and you receive the final report.
- If relevant, we train your team in the process for future studies.

How the remote warehouse mapping service works
The mapping service also comes in a remote version. Our experts still take care of everything from protocol to the final report – your team simply places the data loggers in the warehouse following our guidance.
- One specialists scope the project toghether with your team.
- Our validation team develops the protocol, and you review and sign off.
- You install the loggers according to the protocol and under guidance from our specialists.
- Our team analyze your mapping data and develops the report.
- You receive the final report along with any recommendations based on the findings.
How the temperature mapping kit works
Perform your mappings internally using our GxP-compliant mapping kit with professional-grade equipment, specialized validation software, and access to specialist guidance - if you need it.
- Consult our specialists: Our experts help define the ideal equipment setup for you.
- Plan the study: Create digital test plans and logger placement.
- Set up in minutes: Receive the kit, place the equipment, and connect all loggers with a click.
- Track the mapping: Track the process and act on issues right away with live monitoring.
- Create a digital report: Analyze data, create mapping-optimized graphs, and generate digital reports with in the software.
- Return the kit: Once done, return the kit with the included label.
- Exit calibration is done: We conduct exit calibration, and you can access certificates in the software.

Proven in healthcare logistics
Eupry is trusted by leading logistics companies such as FedEx, WFS, DSV, and many more to validate their global warehouses. They choose Eupry for:
- Speed of deployment – large-scale studies with minimal disruption
- Continuous mapping – no more re-mapping worries across global logistics networks
- Scalable compliance – harmonized SOPs and reports across global sites
Great service! The Eupry Team listens and provides a rapid response. That, added to their top-notch technology, provides accurate mapping/monitoring within industry rules and regulations and peace of mind.
Yolanda Rodriguez, Executive Operations Administrator, FedEx

Included calibration with no idle time
ISO 17025 accredited calibration is included in mapping equipment. Our on-the-wall calibration technology means no downtime waiting for calibration labs.
95% faster than traditional calibration:
- All equipment come pre-calibration
- No equipment removal required
- No data gaps during calibration
- Full traceability maintained

Client Testimonials
How DSV conduct GDP warehouse mapping with Eupry
Before DSV Sweden could utilize their new healthcare logistics warehouse, they needed to ensure its ability to maintain temperature conditions in a way that was both swift and GDP compliant.

The sensors
The sensors work as easily-changeable calibration “antennas” that allow you to calibrate without switching devices – making it the most cost-effective calibration solution on the market.
Tip! Can’t find what you are looking for? These are just examples; see all options in the catalog.
The solution offers sensors for all temperature mapping requirements - here are some examples:

P1T - External Temperature Sensor
- Operating range: -50°C to +50°C (-58°F to 122°F)
- Resolution: 0.01°C (0.018°F)

P1TH - External Temperature & Humidity Sensor
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)
- Extended humidity range: 0-99% RH (Non-condensing)

P1CTH - External CO2, Temperature & Humidity Sensor
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)

P2T - External Temperature Probe
- Operating range: -90 °C to +50 °C (-130 °F to 122 °F).
- Resolution: 0.03 °C (0.054 °F)

P2TH - External Temperature & Humidity Probe
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)
- Extended humidity range: 0-99% RH (Non-condensing)

HC2A - High Precision Humidity Probe
- Accuracy @23 °C: ±0.5 %rh
- Application range: -50 to 100 °C
- Sensor element: HYGRIMER HT-1
- Long-term stability: <1 %rh per year with clean air
See all sensor and data logger options in the product catalog
See how the warehouse mapping solution works and find all product options in our free catalog.
- Technical specifications
- All sensor options
- Solution packages

Monitoring, continuous mapping, and calibration in ONE platform
Eupry is more than mapping. We have created one solution for all your temperature compliance mapping to monitoring and calibration – all in one GxP-compliant platform.
- Reduce total cost of ownership
- Eliminate waste of time spent on manual tasks
- Gain full control (and GDP-compliance) across sites
We have saved around 50-70% of the time we spent on temperature monitoring.
Anders Rasmussen, Senior Logistics Manager, Dechra Pharmaceuticals

Get started with your GDP mapping service
Download our catalog to see the options and technical specifications – or talk to one of our specialists to get answers, guidance, or a tailored quote for your project.









