Guide to winter temperature mapping
Jakob Konradsen


Temperature mapping blueprint
Get a detailed guide of how to conduct reliable temperature mappings of different equipment and units.

Temperature mapping solutions built for GMP and GDP
Learn how the GxP-compliant mapping kit, service, and software works in our solution catalog.
Winter (mapping) is coming. How — and when — should you conduct winter thermal mappings?
In many cases, mapping during winter is a compliance essential. Dive into the guidelines for winter temperature mapping for GMP, GDP, and other highly regulated industries.
Why it is important, whether you need it, and how to do it.
The article outlines:
Why is temperature mapping during winter important?
In reality, it is pretty simple: Winter brings not just festive cheer but also fluctuating external temperatures and humidity levels.
These conditions may fall outside the standard operating conditions and can affect your controlled environments, compromising the quality of products.
Regulatory agencies such as the FDA and EMA require companies to demonstrate the ability to maintain the recommended conditions for products in all (realistic) circumstances, which of course includes winter.
As a result, ignoring winter-specific temperature mapping can result in product spoilage, financial loss, and non-compliance.
Also read: 4 key players you should include in your temperature mapping study
How to perform a reliable temperature mapping
Temperature mapping is complex and requirements vary based on your specific conditions.
These are the main steps that all mappings should entail:
-
Plan ahead: Creating a robust protocol is the cornerstone for compliance and reliability. A well-thought-out plan sets the stage for accurate and repeatable results, eliminating ambiguity in execution.
-
Select equipment: Choose calibrated data loggers that meet your industry standards. Ensure the system allows for easy data extraction, such as report exporting within defined timeframes, to streamline your post-study analysis. Psst.. If you’re looking for equipment, you can see what our specialized, GxP-compliant mapping kit contains here.
-
Execute the study: Follow your protocol to place data loggers at predetermined points. Run the test for the specified period to ensure that you are collecting meaningful and consistent data.
-
Analyze data: Evaluate the collected data to assess temperature consistency across the mapped area. Look for hot or cold spots, and assess whether the facility maintains temperature within the required parameters.
-
Review and adapt: After data analysis, make the required adjustments to your storage settings, procedures, or even the physical layout. If significant changes are needed, be prepared to re-map to validate the new conditions.
Also read: How to choose temperature mapping data loggers and equipment
6 winter-specific conditions in temperature mapping
With the conditions of winter come special circumstances to take into account when performing your temperature mapping.
- HVAC performance: Cold external temperatures may affect your HVAC system, causing possible fluctuations that should be accounted for in your mapping exercise.
- Door openings: Winter may mean fewer door openings, which can impact temperature control differently than in warmer seasons.
- Energy consumption: Increased energy use for temperature maintenance during colder months should be considered in your efficiency evaluation.
- Insulation weak spots: Cold weather can highlight areas where your system may be losing heat, requiring special attention during mapping.
- Condensation risks: The transition from cold external to warm internal temperatures may lead to condensation that could affect product quality.
- Seasonal staffing changes: Consider how fewer staff members during the holidays could impact the execution and monitoring of your mapping study.
Also read: Temperature mapping: Tips, frameworks, and pitfalls
5 things you will get out of a digital mapping solution
As you navigate the complexities of highly regulated industries, you might be looking for a solution that makes temperature mapping less complicated.
Eupry’s mapping solution digitalizes and automates previously manual and error-prone tasks giving you full control over the process and reducing risks.
There are many advantages related to (to some extent) digitalizing your temperature mapping process – here are five of them:
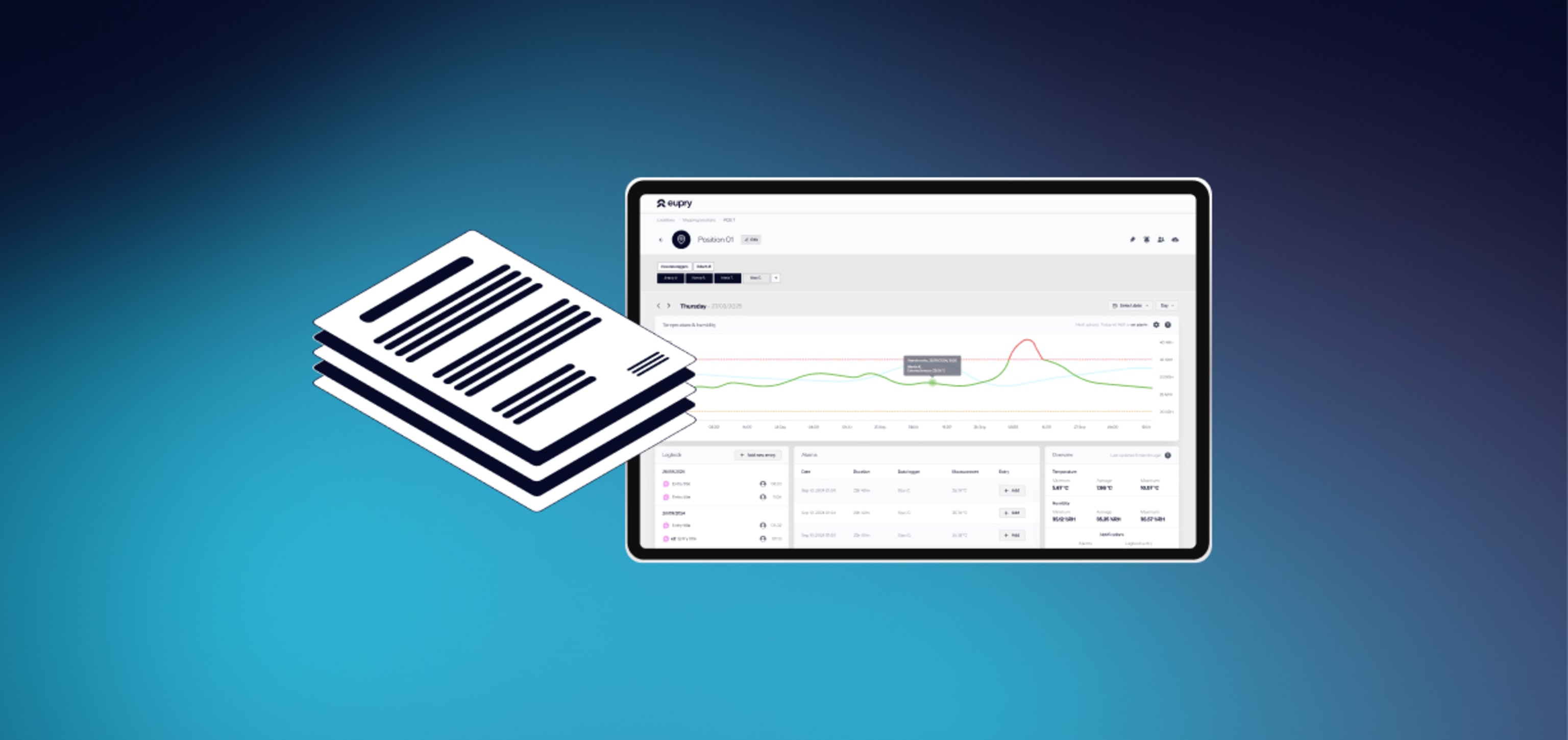
- Full (digital) overview: Get an overview of every step of the process in one digital place. Our specialized mapping software allows you to view, analyze, and export all data easily.
- Live monitoring: Catch issues immediately with live temperature monitoring, minimizing the risk of delays.
- No calibration blocks: Pre-calibrated data loggers and automatically linked certificates in the software will mean no manual pairings and no waiting time.
- Easy digital reporting: Minimize time spent on reporting while securing a more reliable outcome through automated data collection and analysis.
- Compliance certainty: ISO 17025-calibrated, ISO 27001-secured, and FDA 21 CFR Part 11-simplified; rest easy, you are compliant.
Psst.. We also offer (on-site or remote) mapping services where our validation team handles every step of your mapping process. Learn more in our catalog.