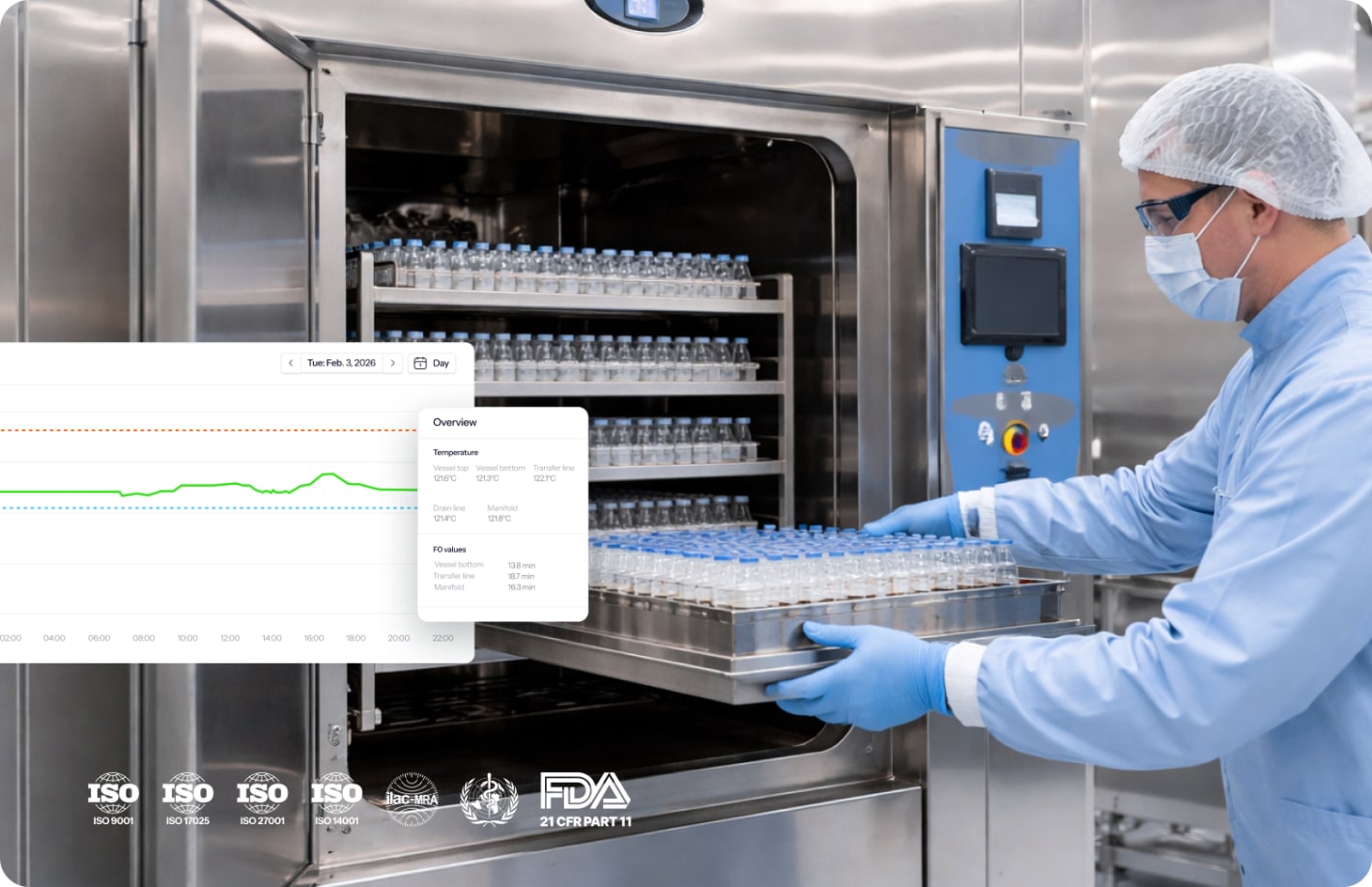
Autoclave validation guide
IQ/OQ/PQ requirements for pharmaceutical facilities

Adam Hartmann-Kruckow
Autoclave validation proves that your steam sterilizer consistently delivers sterility assurance under defined conditions. This guide explains the IQ/OQ/PQ process, regulatory requirements from ISO 17665:2024 and EU GMP Annex 15, and the technical tests – from F₀ calculations to Bowie-Dick procedures – that your need to execute a compliant autoclave qualification.
Get a structured framework aligned with ISO, GMP, FDA 21 CFR 211.113, and EN 285.

Table of contents
- What is autoclave validation, and why is it required?
- Which regulations and standards govern autoclave validation?
- What are the phases of autoclave validation (IQ/OQ/PQ)?
- What are the acceptance criteria for autoclave validation?
- How do you calculate F₀ values for autoclave validation?
- What causes autoclave validation failures and how to fix them?
- How often should autoclaves be requalified?
- What is parametric release for autoclaves, and when is it allowed?
- How do 21 CFR Part 11 and Annex 11 apply to autoclave systems?
- Frequently asked questions about autoclave validation

What is autoclave validation, and why is it required?
Autoclave validation is documented evidence that a steam sterilizer achieves the intended sterility assurance level consistently and reproducibly. The process follows a lifecycle approach covering installation, operational testing, and performance verification under real-world conditions.
Validation matters because sterilization failures create patient safety risks, product recalls, and regulatory consequences. FDA 21 CFR 211.113 and EU GMP require documented proof that sterilization processes work as intended. Without proper validation, companies face warning letters, import alerts, and production shutdowns.
Pharmaceutical QA managers handle 20-50 pieces of equipment requiring periodic requalification. Each autoclave typically needs annual or risk-based requalification studies, consuming validation resources and creating operational bottlenecks. Understanding the validation requirements helps you plan resources, avoid common failures, and maintain audit readiness.
Also read: IQ, OQ, PQ in pharma | Guide to equipment qualification
Which regulations and standards govern autoclave validation?
Multiple regulatory frameworks establish autoclave validation requirements. Understanding which apply to your operations helps you design protocols that satisfy auditors and maintain global compliance.
- ISO 17665:2024 provides the international standard for moist heat sterilization development, validation, and routine control. The 2024 update emphasizes lifecycle approaches, bioburden considerations, and enhanced documentation requirements. This standard applies globally and forms the technical foundation for autoclave validation in pharmaceutical, biotech, and medical device manufacturing.
- EN 285 specifies requirements for large steam sterilizers, including steam quality acceptance criteria. The standard defines limits for dryness fraction (≥0.95), non-condensable gas content (≤3.5% v/v), and superheat (≤25°C above saturation temperature). EN 285 testing verifies that steam has the physical properties needed for effective sterilization.
- EU GMP Annex 15 establishes the qualification and validation framework for pharmaceutical manufacturing in Europe. The regulation requires risk-based approaches, documented change control, and periodic review of requalification intervals. Annex 15 emphasizes that validation is not a one-time event but an ongoing process integrated with quality management systems.
- FDA 21 CFR 211.113 mandates validation of aseptic processing and sterilization. The regulation requires documented evidence that sterilization processes deliver expected results consistently. FDA expects validation to include worst-case scenarios, biological indicator challenges, and demonstrated lethality calculations.
- FDA 21 CFR 211.68 covers automatic, mechanical, and electronic equipment including autoclave controllers and recorders. This regulation requires routine calibration, inspection, and checking according to written programs. Electronic records must meet data integrity requirements for audit trail, user access controls, and secure storage.
- EU GMP Annex 11 governs computerized systems, including autoclave control software and data logging systems. The regulation requires system validation, access controls, audit trails, and backup procedures. Annex 11 compliance proves that electronic temperature and pressure records are trustworthy and tamper-evident.
- PDA Technical Report No. 1 provides industry guidance on validation of moist heat sterilization processe,s including cycle design, biological indicator selection, and ongoing monitoring approaches. While not regulatory, PDA TR 1 represents industry best practices widely accepted by global regulatory agencies.

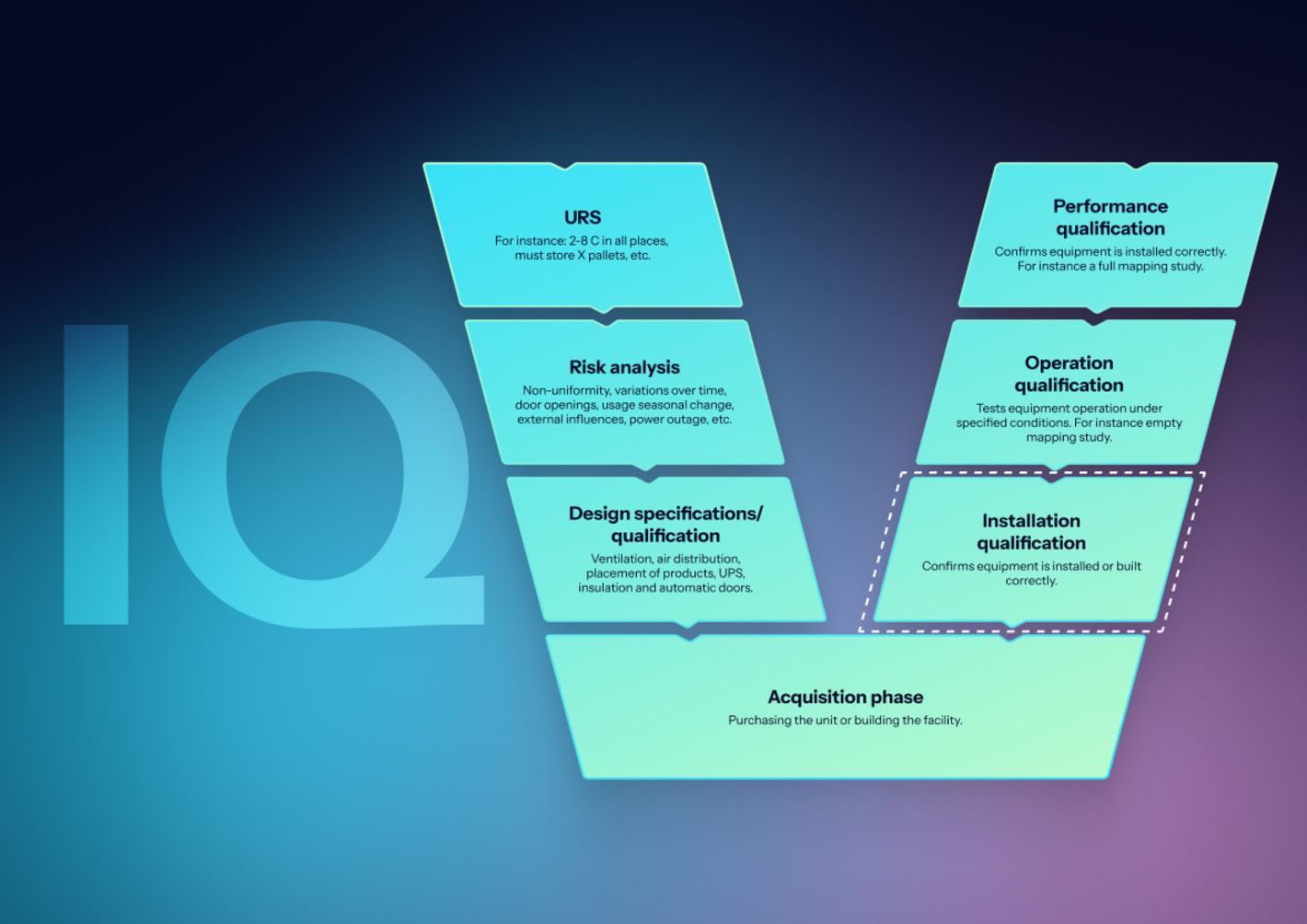
What are the phases of autoclave validation (IQ/OQ/PQ)?
Autoclave validation follows a structured lifecycle approach, moving from design confirmation through installation verification and operational testing to real-world performance demonstration. Each phase builds evidence that the equipment meets specifications and delivers sterility assurance.
What is installation qualification (IQ) for autoclaves?
IQ confirms the autoclave is installed according to manufacturer specifications, design documents match as-built conditions, and all supporting utilities meet requirements. This phase creates the baseline documentation for all future qualification activities.
Equipment documentation review
Verify that all required documents are available and controlled:
- Purchase orders and specifications
- Operation and maintenance manuals
- Piping diagrams and electrical schematics
- Calibration certificates
Missing documentation creates audit findings and complicates troubleshooting during operational issues.
Physical installation verification
Teams confirm that chamber dimensions, utility connections, safety systems, and instrument calibration match specifications. This includes verifying:
- Steam supply pressure and quality
- Compressed air connections
- Electrical supply voltage and phase
- Water supply (if required)
- Drain connections and exhaust ventilation
Deviations between design and installation require investigation and resolution before proceeding.
Safety system verification
Test that all safety systems are present and functional:
- Pressure relief valves
- Door interlocks
- Over-temperature and over-pressure alarms
- Emergency stops
Safety systems protect operators and prevent equipment damage during failures.
Instrumentation verification
Document that all instruments are installed correctly with valid calibration certificates traceable to national standards. This includes chamber temperature sensors, pressure sensors, drain temperature sensors, and other monitoring instruments. Calibration must be current and performed by ISO/IEC 17025 accredited laboratories.
IQ acceptance criteria
IQ acceptance requires:
- All documentation present and current
- Equipment identification matching purchase specifications
- Utilities meeting requirements
- Safety systems functional
- All instruments calibrated
Incomplete IQ creates downstream validation failures and regulatory observations.
Also read: Complete guide to thermal validation

Download a free autoclave validation protocol template
Get a structured framework for qualifying autoclaves in pharmaceutical, biotech, and regulated manufacturing environments, aligned with ISO 17665:2024, EU GMP Annex 15, FDA 21 CFR 211.113, and EN 285.
What is operational qualification (OQ) for autoclaves?
OQ demonstrates that the autoclave operates according to design specifications under no-load conditions. This phase challenges control functions, verifies alarm responses, and establishes baseline temperature distribution before introducing product loads.
Vacuum leak testing (pre-vacuum autoclaves only)
The test verifies chamber integrity by evacuating air, holding a vacuum, and measuring pressure rise rate. Acceptance criteria typically require a pressure rise ≤1.3 mbar/min, though specifications vary by manufacturer. Failed tests indicate door seal problems, piping leaks, or valve failures requiring correction.
Bowie-Dick testing (pre-vacuum autoclaves only)
A standardized test pack with chemical indicators is placed in the chamber center just above the drain. After running a cycle at 134°C for 3.5 minutes, uniform color change confirms complete air removal. Streaking or pale areas indicate air pockets that compromise sterilization.
Three consecutive successful runs are required during OQ. For routine operations, facilities run daily Bowie-Dick tests at the start of the shift. Failed tests trigger investigations and may require revalidation.
Empty chamber heat distribution studies
Temperature mapping uses calibrated sensors at 5-15 locations, depending on chamber size:
- Chamber center
- Four corners
- Near door
- Near drain
- Near steam inlet
Three consecutive cycles must demonstrate:
- All locations maintain 121-124°C (for 121°C cycles)
- Uniformity within ±2-3°C of mean temperature
- Cold spots identified for PQ focus
Poor temperature distribution indicates steam flow problems, inadequate air removal, or equipment malfunction.
Also see: Temperature mapping: Tips, frameworks, and pitfalls
Steam quality testing per EN 285
Three parameters verify that the steam meets the physical requirements for effective sterilization:
Dryness fraction:
- Production equipment: ≥0.95 (95% dry steam minimum)
- Laboratory equipment: ≥0.90 may be acceptable
- Wet steam reduces heat transfer and causes wet pack failures
Non-condensable gas (NCG) content:
- Must not exceed 3.5% v/v
- High NCG indicates steam supply problems or inadequate air removal
- Displaces steam and prevents sterilization
Superheat:
- Must not exceed 25°C above saturation temperature
- Excessive superheat acts like dry heat, reducing effectiveness
- Indicates steam supply or pressure control issues
What is performance qualification (PQ) for autoclaves?
PQ demonstrates consistent sterilization achievement under actual operating conditions with representative product loads. This phase uses both physical measurements and biological challenges to prove sterility assurance.
Worst-case load determination
Identify the most challenging sterilization conditions based on:
- Maximum chamber capacity by weight or volume
- Most difficult items to sterilize (material density, packaging)
- Typical production load patterns
- Combinations of these factors
The load configuration must be justified and documented to prove the autoclave can sterilize under any routine conditions.
Loaded chamber heat penetration studies
Calibrated temperature sensors (minimum 10-15 locations) are placed throughout the load, focusing on cold spots identified during empty chamber studies. Sensors must be in intimate contact with items being sterilized to measure actual product temperatures, not just chamber air.
Three consecutive cycles must demonstrate:
- All load locations reacha minimum 121°C
- Temperature maintained for specified hold time
- Adequate F₀ values calculated from temperature profiles
F₀ calculation:
F₀ = Σ 10^((T-121)/Z) × Δt
Where:
- T = observed temperature
- Z = 10°C for Geobacillus stearothermophilus
- Δt = time interval in minutes
Minimum F₀ of 12 minutes at 121°C represents the industry standard for overkill cycles, though specific targets depend on bioburden-based cycle design and risk assessment.
Biological indicator challenge studies
Geobacillus stearothermophilus spores (typically 10⁶ CFU population) are placed in worst-case locations to verify microbial kill. After cycle completion, indicators are incubated per manufacturer instructions (typically 7 days at 55-60°C) and examined daily for growth.
Acceptance criteria:
- No growth in all exposed indicators (confirms complete sterilization)
- Positive controls show growth (confirms indicator viability)
- Three consecutive successful cycles required
Failed biological indicators trigger full investigations including review of cycle parameters, load configuration, steam quality, and equipment function before revalidation attempts.
Load dryness testing (porous loads)
Items are weighed before and immediately after cycles to calculate moisture gain.
Acceptance criteria:
- ≤1-2% moisture gain
- No visible wetness
Wet packs indicate inadequate drying time, poor steam quality with excessive NCG, improper wrapping materials, or drainage problems.
What are the acceptance criteria for autoclave validation?
Acceptance criteria must be specific, measurable, and based on scientifically sound principles. Vague criteria create interpretation problems during execution and audit defense.
What are the temperature and pressure requirements?
Sterilization temperature range depends on cycle design. For 121°C cycles, all chamber and load locations must maintain 121-124°C during hold time. For 134°C cycles used with pre-vacuum autoclaves, the range is 132-136°C. Temperature uniformity requires all sensors within ±2-3°C of the mean temperature, though tighter tolerances may apply for specific applications.
Hold time begins when the coldest location reaches minimum temperature and continues for the programmed duration. Typical hold times range from 15-60 minutes, depending on cycle design, load type, and sterility assurance level requirements. Timer accuracy must be within ±5% or as specified by the manufacturer.
Pressure correlates to temperature for saturated steam. At 121°C, pressure should be approximately 1.0 bar gauge (2.0 bar absolute). At 134°C, pressure should be approximately 2.0 bar gauge (3.0 bar absolute). Deviations between temperature and pressure indicate non-condensable gases or superheat problems.
What are the steam quality acceptance limits per EN 285?
EN 285 establishes quantitative acceptance limits for steam physical properties. Dryness fraction ≥0.95 for production equipment ensures sufficient latent heat transfer. Some authorities accept ≥0.90 for laboratory equipment, depending on load type and application.
Non-condensable gas content ≤3.5 ml per 100 ml condensate (3.5% v/v) limits air and other gases that displace steam and reduce lethality. Higher NCG indicates steam supply contamination or inadequate air removal.
Superheat ≤25°C above saturation temperature maintains steam condensation properties. Excessive superheat creates dry heat sterilization conditions requiring longer exposure times and higher temperatures.
What F₀ values and biological indicator results are required?
Minimum F₀ values depend on the cycle approach. Overkill cycles typically target ≥12 minutes at 121°C reference temperature, providing conservative sterility assurance without bioburden knowledge. Bioburden-based cycles calculate F₀ from measured bioburden and desired sterility assurance level using:
Biological F₀ = D₁₂₁ × (log N₀ – log N)
Where D₁₂₁ equals the D-value at 121°C for the indicator organism, N₀ equals the initial spore population, and N equals the desired residual probability, typically 10⁻⁶ for sterility assurance level.
Physical F₀ calculated from heat penetration data must equal or exceed biological F₀ requirements. This verification confirms that measured temperatures would achieve the calculated lethality against the biological challenge.
Biological indicators with 10⁶ spore population must show no growth after three consecutive cycles. Positive controls must demonstrate growth, confirming indicator viability. Any increase in test indicators indicates sterilization failure requiring full investigation and revalidation.
What are the vacuum leak and Bowie-Dick acceptance criteria?
Vacuum leak tests for pre-vacuum autoclaves require a pressure rise rate ≤1.3 mbar/min or per manufacturer specification during the hold period. Higher rates indicate seal degradation or piping leaks compromising air removal.
Bowie-Dick tests require uniform color change across the entire indicator sheet with no pale areas or streaks. Non-uniform results indicate inadequate air removal, creating cold spots that prevent steam penetration. Three consecutive successful tests establish baseline performance with daily testing demonstrating continued effectiveness.
Also see: Understanding acceptance criteria in temperature mapping
How do you calculate F₀ values for autoclave validation?
F₀ calculation integrates the lethal effect of temperature over time, converting variable temperature profiles to equivalent minutes at 121°C reference temperature. The calculation uses the relationship that a 10°C temperature increase doubles lethality for Geobacillus stearothermophilus spores.
The formula F₀ = Σ 10^((T-121)/10) × Δt applies at each time interval where T equals measured temperature in °C and Δt equals time interval in minutes. Modern data acquisition systems calculate F₀ automatically, but validation engineers must understand the underlying methodology to verify results and troubleshoot discrepancies.
For manual calculation, temperature data recorded at regular intervals, such as every minute, enables summation of lethal contributions. Temperatures below 100°C contribute negligible lethality and are often excluded from calculations. The coldest probe location determines the minimum F₀ for the cycle.
Example calculation: A sensor records 115°C for 5 minutes, 121°C for 20 minutes, and 124°C for 10 minutes. The F₀ contributions are:
- At 115°C: 10^((115-121)/10) × 5 = 10^(-0.6) × 5 = 0.251 × 5 = 1.26 minutes
- At 121°C: 10^((121-121)/10) × 20 = 10^(0) × 20 = 1.0 × 20 = 20.0 minutes
- At 124°C: 10^((124-121)/10) × 10 = 10^(0.3) × 10 = 1.995 × 10 = 19.95 minutes
Total F₀ = 1.26 + 20.0 + 19.95 = 41.21 minutes
Multiple probe calculations require evaluating F₀ at each location. The minimum F₀ value across all probes must meet or exceed the acceptance criteria. If any location falls below the minimum F₀, the cycle fails validation and requires investigation.
Z-value selection affects calculation sensitivity. The standard Z-value of 10°C for G. stearothermophilus reflects the organism's heat resistance characteristics. Different organisms require different Z-values, but 10°C applies to most pharmaceutical steam sterilization applications.
What causes autoclave validation failures, and how do you fix them?
Validation failures waste time, delay production, and consume resources. Understanding root causes enables targeted troubleshooting and faster resolution.
What causes wet pack failures in autoclaves?
Wet packs result from multiple causes. Excessive non-condensable gases prevent complete steam penetration, leaving residual moisture. Testing steam quality per EN 285 identifies NCG problems requiring steam supply improvement or maintenance. Inadequate vacuum in pre-vacuum cycles leaves air pockets that condense as liquid during cooling. Vacuum leak testing and vacuum pump maintenance address these issues. Poor drainage from improper load positioning allows condensate pooling. Load configuration modifications improve drainage.
Insufficient drying time fails to evaporate residual moisture before door opening. Extending the drying phase or reducing the end-of-cycle pressure rise rate reduces wetness. Incorrect wrapping materials that absorb moisture or block steam penetration require evaluation and replacement with validated materials.
Why do Bowie-Dick tests fail?
Failed Bowie-Dick tests indicate air removal problems in pre-vacuum cycles. Insufficient vacuum pulses or leaks in chamber seals allow air retention, creating cold spots. Increasing pulse number, extending pulse duration, or replacing door gaskets and penetration seals resolves mechanical issues.
Non-condensable gas in the steam supply displaces the air removal capacity. Steam quality testing identifies supply problems requiring boiler maintenance or steam trap servicing. Chamber drain blockages prevent condensate removal, interfering with vacuum formation. Regular drain cleaning prevents accumulation.
Incorrect test pack positioning affects results. Bowie-Dick packs must be placed flat in the chamber center just above the drain point per the test procedure. Variations in placement create inconsistent results, not reflecting actual air removal performance.
What causes biological indicator failures?
BI failures indicate insufficient lethality at the placement location. Cold spots from poor steam distribution require adjustment of steam injection points, baffle positioning, or load configuration. Inadequate hold time at temperature requires cycle parameter modification with revalidation.
Instrument calibration drift causes temperature reading errors. Recalibration of chamber sensors and validation of thermocouples with traceable standards corrects measurement problems. Steam quality deficiencies, including wet steam, NCG contamination, or excessive superheat, reduce sterilization effectiveness, requiring steam supply correction.
Incorrect BI placement away from worst-case locations provides false confidence. BIs must be positioned in identified cold spots from heat penetration studies. BI handling errors, including exposure to heat or humidity before use, contaminated indicators, or expired product,s create false positives requiring confirmation with fresh indicators.
What causes temperature uniformity problems?
Non-uniform temperature distribution indicates steam flow or air removal issues. Poor air elimination leaves pockets that resist heating. Enhanced vacuum cycling or extended conditioning time improves air removal. Blocked steam inlet filters or spray nozzles reduce steam delivery. Regular filter cleaning and nozzle inspection maintain performance.
Overloading the chamber beyond the validated capacity restricts steam flow, creating cold spots. Adherence to validated load configurations prevents this problem. Insufficient steam supply pressure or flow rate prevents proper conditioning. Verification that utilities meet autoclave requirements and correction of deficiencies solves supply problems.
Malfunctioning temperature sensors provide inaccurate data. Cross-checking chamber sensors against calibrated validation instruments identifies sensor problems requiring replacement. Failing to allow adequate conditioning time before the hold phase starts leaves some locations below the temperature. Extending pre-heat or conditioning phases ensures all areas reach temperature before timing begins.
Also read: Steam-in-place (SIP) validation guide
How often should autoclaves be requalified?
Requalification frequency depends on risk assessment, change control, and regulatory requirements rather than arbitrary calendar intervals. EU GMP Annex 15 emphasizes risk-based approaches where companies justify requalification schedules through documented review of equipment performance, maintenance history, deviation trends, and process criticality.
How do you determine risk-based requalification intervals?
Many facilities default to annual requalification, but longer intervals are defensible with proper justification. Factors supporting extended intervals include robust continuous monitoring showing stable performance, minimal deviations or excursions, a comprehensive preventive maintenance program with documentation, regular calibration of instruments, no significant equipment modifications, and documented periodic review of performance data.
Conversely, shorter intervals or immediate requalification are needed when monitoring shows performance drift, repeated cycle failures occur, major repairs or parts replacement happen, significant process changes occur, audit findings or regulatory observations are received, or relocation of equipment takes place.
Documentation of the risk-based decision process proves compliance. This includes review of deviation logs, calibration records, maintenance history, and performance trends. QA must approve the requalification schedule, and change control procedures must trigger requalification when predefined events occur.

What changes trigger mandatory requalification?
Certain changes always require requalification regardless of the scheduled interval. Major repairs to the chamber, door seals, or steam system alter equipment characteristics, potentially affecting performance. Replacement of control systems, sensors, or recording devices changes the equipment, requiring reverification.
Changes to validated cycle parameters, including temperature, pressure, or hold time, need revalidation to prove continued sterility assurance. Introduction of new load configurations not previously validated requires worst-case determination and PQ studies. Equipment relocation, even within the same facility, necessitates IQ and potentially OQ revalidation due to utility differences.
Repeated cycle failures or deviations suggest underlying problems requiring investigation and revalidation, even mid-interval. Negative trend analysis showing gradual performance degradation triggers evaluation and potential requalification before the scheduled date. Regulatory inspection findings specifically requiring revalidation mandate immediate action regardless of the last qualification date.
What documentation is required for requalification?
Requalification follows the same IQ/OQ/PQ structure as initial validation, though the scope may be reduced based on risk assessment. IQ may be abbreviated if equipment has not been moved and utilities remain unchanged. OQ typically includes full empty chamber studies and alarm verification. PQ must demonstrate performance with current load configurations. Protocols must reference previous validation data, establishing continuity and documenting changes. Acceptance criteria remain consistent with initial validation unless justified modifications occur. Deviation procedures must address any failures during requalification, providing root cause analysis and corrective actions.
Final reports require QA approval before releasing equipment to routine operation. The requalification study updates the equipment validation status and resets the next review date based on approved intervals.
What is parametric release for autoclaves, and when is it allowed?
Parametric release allows the release of sterilized products based on process data instead of sterility testing. EU GMP Annex 17 establishes the framework requiring demonstrated process capability, comprehensive validation, and robust monitoring.
What are the requirements for parametric release?
The sterilization process must be extensively validated with documented capability to achieve the required sterility assurance level consistently. This includes heat distribution studies, heat penetration studies, biological challenge studies, and demonstration of cycle reproducibility over extended periods.
Continuous monitoring of critical parameters, including time, temperature, pressure, and steam quality, must prove that the process operates within validated ranges. Automated recording with data integrity controls per Annex 11 provides reliable documentation. Sensors must be calibrated regularly with evidence of accuracy.
Process deviations require investigation and potentially product rejection. Any excursion outside validated parameters necessitates a case-by-case evaluation of product sterility assurance. Trend analysis identifies gradual process drift before reaching action limits.
When is parametric release appropriate for autoclaves?
Terminal sterilization of sealed containers commonly uses parametric release because product sterility testing is destructive and statistically limited. Large batch sizes make sterility testing of representative samples impractical. Well-controlled processes with extensive validation history build confidence for parametric approaches.
Porous load sterilization rarely qualifies for parametric release due to complexity and variability. Load configuration, wrapping materials, and item density affect sterilization effectiveness, making process control more challenging. Biological indicators often remain the primary release method for these applications.
New products or load configurations require traditional sterility testing and biological challenges until sufficient process knowledge develops. Regulatory acceptance varies by region, with some authorities requiring continued periodic biological indicator testing even under parametric release programs.
What are the regulatory considerations for parametric release? Implementation requires regulatory approval or notification, depending on jurisdiction. Documentation must demonstrate that validated process parameters guarantee sterility, meeting statistical confidence levels. Quality risk management per ICH Q9 justifies the parametric approach and establishes appropriate controls.
Annual product quality review must include sterilization performance data, deviation trends, and validation status. Any indication of reduced process capability triggers investigation and potential return to traditional release methods until corrective actions restore performance.
How do 21 CFR Part 11 and Annex 11 apply to autoclave systems?
Computerized autoclave control systems generate electronic records subject to FDA 21 CFR Part 11 and EU GMP Annex 11 data integrity requirements. These regulations ensure electronic data is reliable, attributable, and audit-ready.
What are the data integrity requirements for autoclave electronic records?
Electronic records must be attributable to specific operators through unique user IDs and secure authentication. Shared logins or passwords violate data integrity principles. Systems must enforce password complexity and expiration policies, preventing unauthorized access.
Complete and accurate records require capturing all data relevant to batch release, including cycle parameters, alarm events, operator actions, and deviations. Deleted or overwritten data without documentation creates compliance issues. Audit trails must be enabled, recording who accessed or modified records, what changes occurred, when changes were made, and why modifications happened.
Consistent data across the lifecycle requires secure archival for minimum retention periods typically 5-10 years, depending on product type and regulatory requirements. Backup and disaster recovery procedures protect against data loss. Electronic signatures must meet requirements for non-repudiation, proving signer identity.
Available records must be readable throughout the retention period, regardless of system changes. Migration to new systems requires validation and verification of data integrity. Metadata preservation proves record authenticity and chain of custody.
What validation is required for autoclave computerized systems?
Autoclave control systems and data acquisition software require validation proving they function as intended and meet user requirements. Validation scope includes software functionality, interface controls, data integrity controls, and security features.
System validation documentation includes:
- User requirements specification
- Functional specification
- Validation protocols and executed test records
- Traceability matrix
- Validation summary report
Deviations during validation require investigation and resolution before system release.
How should hybrid paper-electronic systems be handled?
Many facilities operate hybrid systems combining electronic cycle control with paper records or manual data transcription. These arrangements create data integrity risks, including transcription errors, delayed documentation, and a lack of an audit trail. Regulatory agencies increasingly challenge hybrid approaches, especially where electronic systems exist but paper records are used for release decisions.
Full electronic implementation provides better compliance and operational efficiency. If hybrid systems persist, procedures must define which record is primary, how discrepancies are resolved, and how electronic data is secured and retained. Regular review of the hybrid system justification determines when full electronic implementation becomes necessary.

Download the autoclave validation protocol template
Get immediate access to our comprehensive IQ/OQ/PQ protocol template with acceptance criteria, test procedures, and documentation frameworks aligned with ISO 17665:2024, EN 285, and GMP Annex 15 requirements.

Frequently asked questions about autoclave validation
Autoclave validation services
From qualification to continuous GMP/GLP compliance
Eupry provides comprehensive validation, continuous monitoring, and calibration solutions for pharmaceutical and biotech facilities - from autoclaves to TCUs and warehouses.