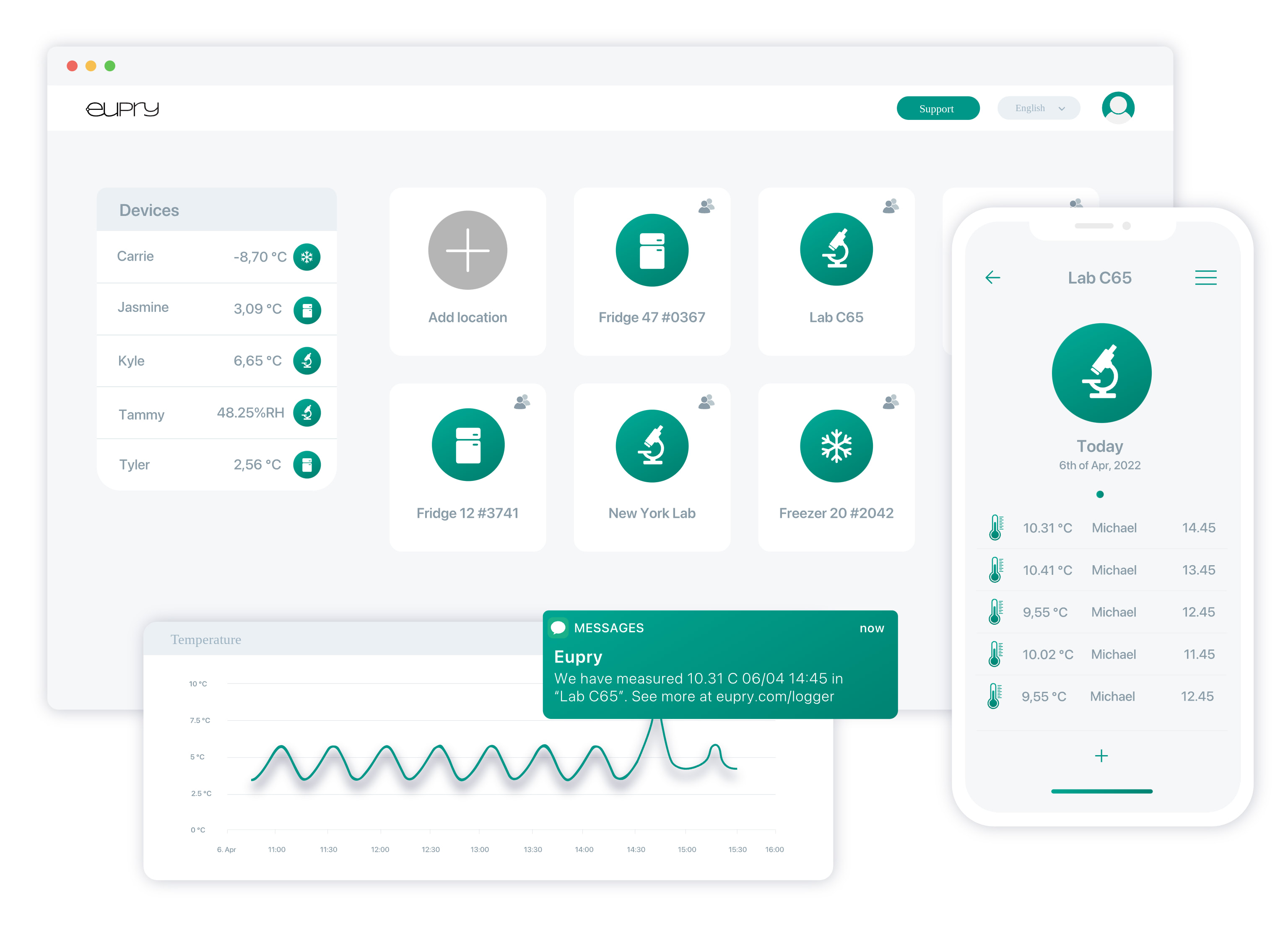
How the monitoring software works
Watch real-time and historic measurements in a user-friendly, IT-validated online environment. In the software, you can, for instance, find

Customers worldwide benefiting from Eupry

Gain complete certainty that everything just works (compliantly)
Tailored for laboratories
The service provides you with dependable, cost-effective, real-time temperature and humidity monitoring tailored to the diverse needs of laboratories.
The ISO-accredited solution is suitable for all types of cargo – including highly regulated pharmaceutical products.

Don't take our word for it
"We have saved around 50-70% of the time we spend on temperature monitoring."
Anders Rasmussen Senior Logistic Manager, Dechra Pharmaceuticals PLC
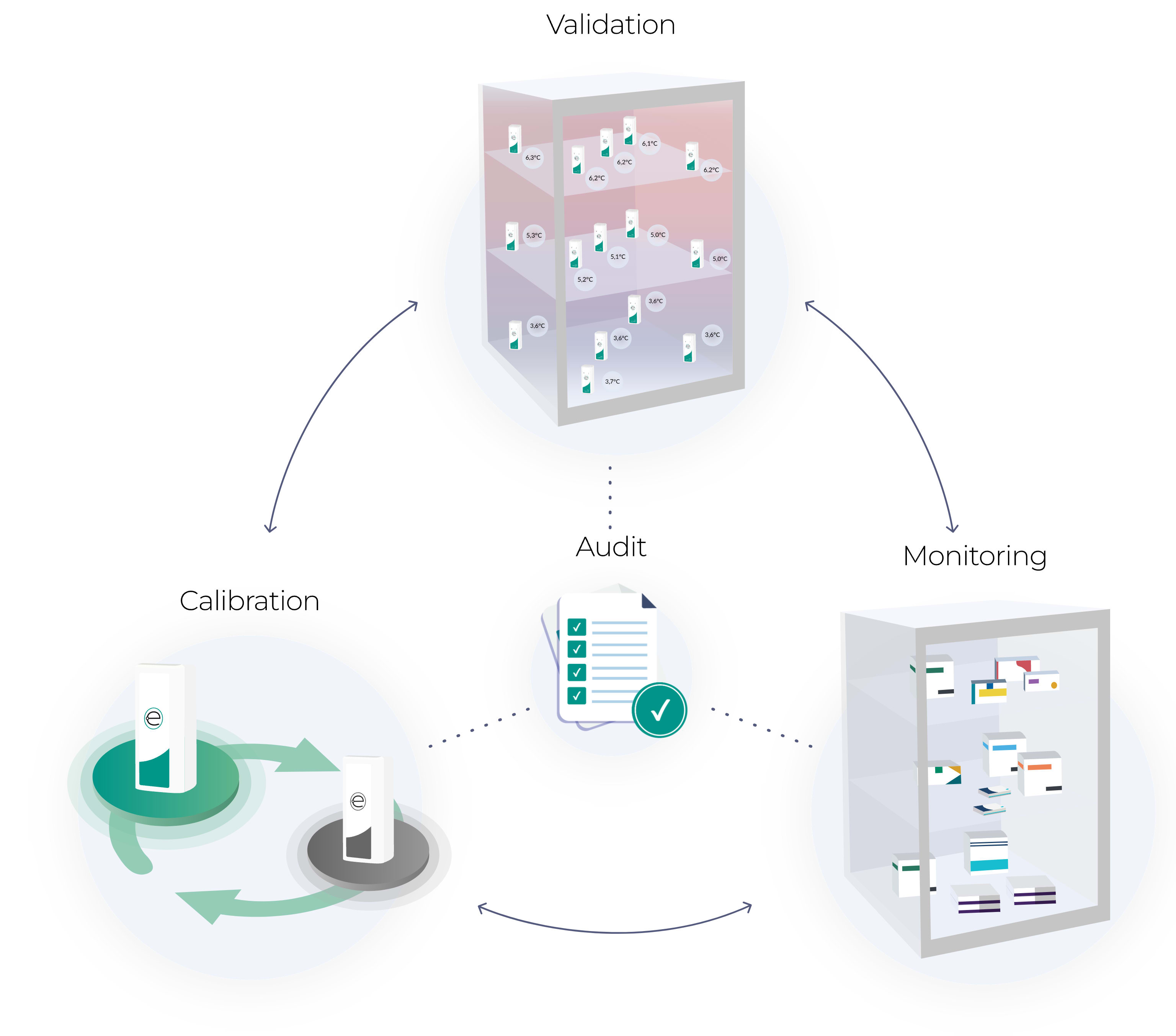
Eupry is more than monitoring.
It is a digital system and water-tight process that gives you full control over all your temperature compliance efforts and collects all data in a single view – from temperature monitoring to data logger calibration and mappings – to make sure nothing falls between the cracks.
Less time sifting through the mess
One-click reporting, easy access to all the data you need, and digital storage of ALL your temperature data – from calibration certificates to temperature records and deviations. With everything accessible, it is simple to find the information that matters most.
Be on the forefront of compliance requirements
The temperature monitoring solution makes worrying about changes in the ever-increasing compliance landscape a thing of the past.
Our industry experts make sure the service evolves with the compliance landscape to always keep you one step ahead on the compliance ladder.
The (not so) obvious route made obvious
From clients to colleagues: Rocking the boat and getting everyone onboard with new ways of working can be tricky.
But the simplicity of the temperature compliance solution makes it not only easy to implement and use, it also makes it easy to grasp why the new route is actually the only right way to do things.
(And should someone need a helping hand, our specialists are ready to step in).
That's cool, but what are the benefits?
Curious about the specifics?
Enjoy audits
With Eupry, you can export all required auditing data in three clicks.
Minimize mistakes
Removing mundane tasks means removing manual errors. We believe in compliance confidence.
Full process clarity
Complete overview of every step of the monitoring process. Control the process to control the quality.
Obviously easy
Why has temperature compliance become so complex? According to our customers, the solution is easy to learn, use, and pitch internally.
Someone to call
Questions? Our temperature compliance specialists are on call when you need.
Financial certainty
Get complete predictability through a subscription model and full equipment warranty. If it breaks, we will replace it (but it won’t break).
Adapt to your needs
Subscription flexibility lets you scale when you – or your management – say it is time to expand on equipment or locations.
No compliance surprises
ISO 17025-calibrated, ISO 27001-secured, and FDA 21 CFR Part 11-simplified – rest easy, you are fully compliant.
Free and automated calibration without swapping data loggers
Our patented calibration technology offers notable time savings by automating the process and eliminating the need for replacing your data loggers.
The system monitors your loggers’ calibration status and when it’s time, we simply send you newly calibrated sensors to replace the old ones.
Forget spare loggers and their limitations, calibrate all your gear at once, and leave behind the stress of tracking calibration statuses. Of course, you can also get an overview of your logger’s status with a few clicks.
But you don’t have to. We’ve got your back.
1. You are notified
The system monitors your data loggers’ status, notifies you when it’s calibration time, and we send you newly calibrated sensors. No more worrying about keeping up with calibrations.
2. You replace sensors in seconds
The patented technology allows you to simply replace the sensor in each data logger without ever having to remove them from the unit – all your calibrations are now done.
3. Documentation is stored
The software automatically joins data, and calibration certificates are digitally stored and can be accessed and included in an audit-ready report with just a few clicks.
High compliance rating by Novo Nordisk
Eupry Aps provided (…) documentation to conclude that procedures and efficient controls to ensure compliance are in place and well-functioning as intended in all key areas/processes (…)
ISO AUDIT REPORT by Novo Nordisk, June 2023

How it works
The compliance components
All your compliance boxes? Consider them ticked. Plus, we keep you ahead as requirements develop.
“A short way of describing our experience with the solution is simply that it is really easy to use. Both to get started and to work with in day-to-day operations.”
Dorte Egeberg & Allan Toft Jacobsen, Lab Specialist & COO at European Sperm Bank
Client Testimonials
Client Testimonials
Client Testimonials

Get your free product catalog
See all the technical details about how the temperature compliance solutions work.
Dependable temperature compliance for laboratories
Reduce risks, manual work, and costs with reliable temperature monitoring, mapping, and calibration designed for labs.
- Print audit reports in 3 clicks
- Reach quality experts 24/7
- Calibrate without swapping devices


Smarter temperature mappings
Use our mapping solutions to save time, cut costs, and heighten your temperature mapping study quality.
Go hands-on with a leased mapping kit and the specialized mapping software or let our specialists take care of everything for you.
With the mapping solution, you will be able to:
- Identify issues instantly to reduce risks and costly delays.
- Generate a full report tracking the study.
- Easily review certifications before the mapping.














