What are the WHO’s guidelines for temperature mapping?
The World Health Organization’s (WHO) guidelines for temperature mapping are among the most recognized standards in GxP-regulated industries like pharma, biotech, and logistics – and for good reason.
Following the guidelines can help you streamline temperature compliance and demonstrate that your organization is up-to-date with current best practices.
But what do they entail? And how do you (most efficiently) implement them?
This article breaks down WHO’s mapping approach to help you put the guidelines into action for your next mapping study.


8-step checklist: WHO’s temperature mapping guidelines
Get a step-by-step guide to implementing the principles in our WHO temperature mapping guideline checklist.
On this page, we will go through:
- What are the WHO temperature mapping guidelines all about?
- Why should you use WHO’s guidelines for temperature mapping in GxP?
- In which industries are WHO’s mapping guidelines relevant?
- Which mapping guidelines do the WHO offer?
- What are the key parts of WHO’s mapping guidelines (and how to implement them)?
- How do the WHO mapping guidelines align with other guidelines?
What are the "WHO temperature mapping guidelines" all about?
Temperature mapping is part of the validation process aimed at verifying that manufacturing, storage, and transportation environments can maintain the temperature conditions necessary to protect the products or samples they hold – and the WHO’s temperature mapping guidelines have been established to help organizations conduct these study in a safe, reliable, and consistent manner.
The guidelines provide methodologies for assessing risks, such as identifying critical control points in the storage and transportation processes. Implementing WHO standards can help you preemptively address potential issues affecting product quality and safety, thereby reducing the likelihood of costly compliance failures.
Learn more about temperature mapping and why it is important.

Why use WHO’s guidelines for temperature mapping in GxP?
The WHO guidelines play a pivotal role in establishing standards for temperature mapping within regulated Good Practices (GxP) environments, such as pharmaceuticals, biotechnology, and healthcare.
Here are some reasons why WHO’s guidelines are relevant for GxP:

1. It is a globally respected authority
The WHO is vastly recognized worldwide and sets a universally recognized benchmark for maintaining product integrity across borders. In other words, compliance with WHO guidelines can significantly enhance your organization's credibility within the global marketplace.

2. It lives up to auditor expectations
The WHO guidelines are accepted by both governmental and customer auditors. Regulators, business partners, and customers recognize adherence to WHO standards as a commitment to quality and safety. As Jakob Konradsen, Eupry’s co-founder and Chief Quality Officer, stated in our webinar on the topic, “If you adhere to the WHO guidelines, you should be out of the bush in terms of being compliant.”
3. It forms the basis of universal standards
WHO guidelines offer a framework that is often aligned with or forms the basis for other international regulatory requirements. By adhering to WHO standards, you can ensure that you meet not only local regulatory demands but also international best practices and maintain a consistent approach to quality across markets.

4. It is kept up to date
WHO guidelines are continuously reviewed and updated to reflect the latest scientific, technological, and regulatory advancements. By integrating the latest WHO recommendations, you can thereby ensure that you stay competitive in your industry and that your products consistently meet high-quality standards.
5. It is free
Unlike, for instance, ISP guidelines, the WHO temperature mapping guidelines are available at no cost, reducing the threshold for starting to use them.
In which industries are WHO’s mapping guidelines relevant?
The WHO’s emphasis on detailed temperature mapping as part of a validation approach is especially relevant in sectors where precise temperature control is necessary to maintain product or sample integrity and safety.
For example:
- Pharmaceutical companies: Ensuring drugs are stored and transported within safe temperature ranges to maintain quality and efficacy is crucial for pharma companies, which are among the most highly regulated in the world. In other words, WHO guidelines are relevant for compliance with regulatory standards and ensuring patient safety.
- Biotechnology firms: Stable environmental conditions are necessary for biotech companies, whose products often include biologically derived materials. The WHO guidelines help ensure that these sensitive materials are not exposed to temperature variations that could affect their characteristics.
- Logistics companies: Companies specializing in the logistics of temperature-sensitive goods, such as pharmaceuticals, must also adhere to temperature guidelines to prevent product loss. The WHO guidelines can provide a basis for developing solutions that maintain product integrity throughout the supply chain.
- Healthcare providers: Hospitals and other healthcare facilities handle and store temperature-sensitive pharmaceuticals. Adhering to WHO guidelines can help these providers manage their storage environments to ensure that treatments delivered to patients are safe and effective.
- Food and beverage manufacturers: While not directly regulated under the same stringent standards as pharmaceuticals, food and beverage companies can also benefit from following WHO guidelines to prevent spoilage and ensure consumer safety, especially in the handling and shipping of perishable goods.
Also read: Key guidelines and compliance regulations of temperature mapping
Which mapping guidelines do the WHO offer?
First of all, WHO has defined the principles required for the storage and transportation of Time and Temperature Sensitive Pharmaceutical Products (or TTSPPs), based on international regulations and best practices, in WHO Technical Report Series, No. 961, Annex 9 (2011): Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products.
In regards to the temperature mapping studies of TTSPPs, WHO has issued two additional supplements on Annex 9, Section 4.7: Qualification of temperature-controlled stores, listed below:
-
WHO Technical Report Series, No. 992, Annex 5, Supplement 7 (2015), Qualification of temperature-controlled storage areas: Supplement 7 lays out the requirements a controlled-temperature facility or unit needs to follow prior to its use. The report acts as a guide on how to perform the Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) that need to be conducted before the use of the unit.
-
WHO Technical Report Series, No. 992, Annex 5, Supplement 8 (2015): Temperature mapping of storage areas: Supplement 8 states the purpose of the temperature mapping study and counsels on how to execute a temperature mapping in a temperature-controlled area. It gives information on the positioning of mapping sensors, on the acceptance criteria that need to be followed, the structure of the mapping report, and the way data should be analyzed.

What are the key parts of WHO’s temperature mapping guidelines (and how do you implement them)?
The WHO mapping guidelines are designed to be thorough yet flexible enough to apply to different technologies and facilities — this duality can, however, make them tricky to navigate in practice.
The following provides a high-level overview of the WHO guidelines' fundamental parts. Note that it is a distilled summary of the guidelines aimed at making the guidelines more comprehensible. Find the full set of guidelines on WHO’s website.
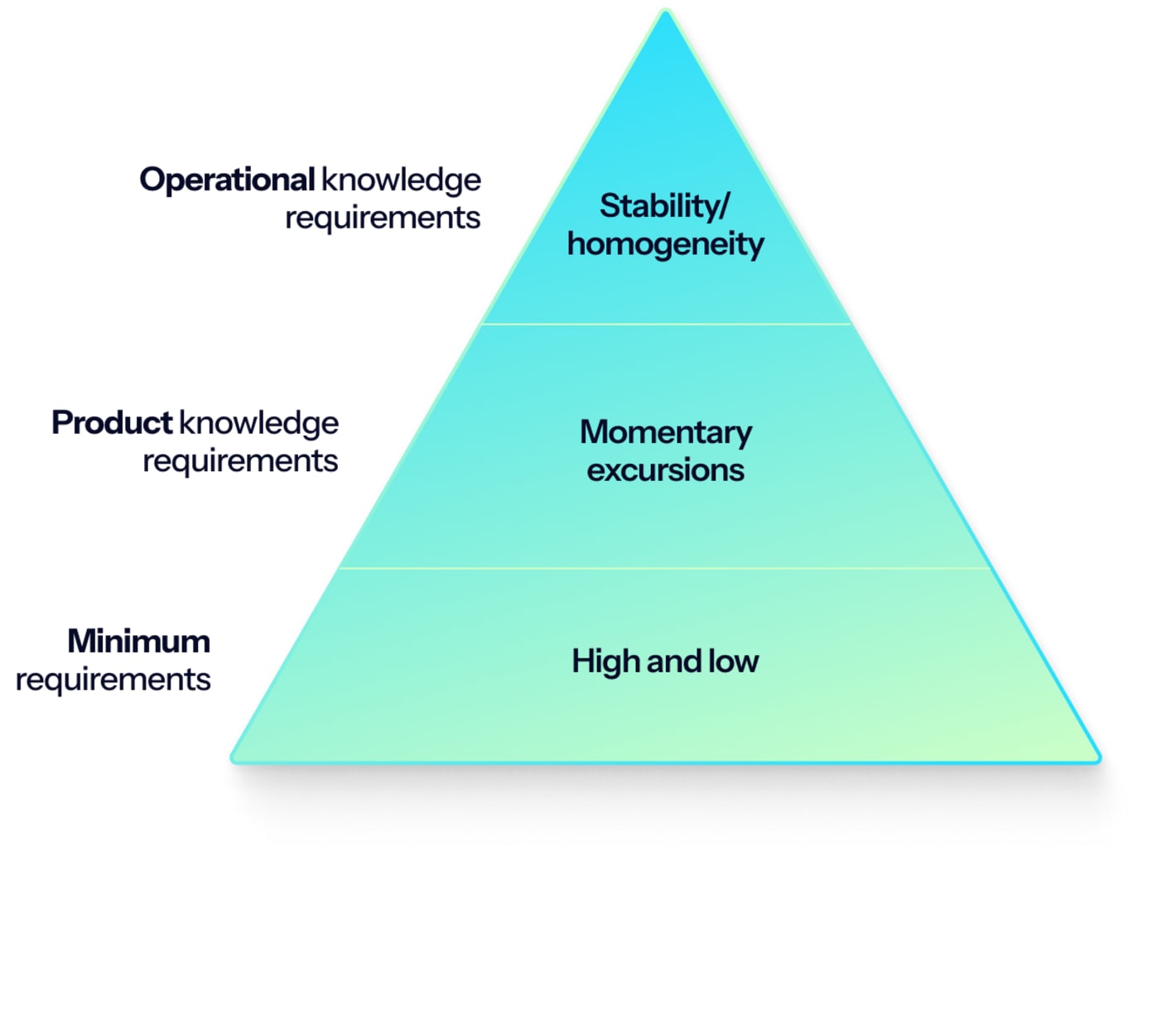
1: Know the requirements of your facility
Requirements are very important when it comes to conducting GxP temperature mappings. We see a lot of uncertainty about this topic. What are the requirements for a WHO-abiding mapping process? What will an auditor expect you to know and take into account?
Note: The model is our own visualization and not part of the WHO guidelines.
Tip! It is essential that you can show auditors that you know your requirements now and that you knew what they were before you started the mapping. We have seen organizations getting deviations because they did not know their requirements in advance.
Tier 1: Minimum requirements
High and low critical values are the bare minimum requirements you should know. What are the high and low critical temperature values we can accept for the product we are storing?
Tier 2: Product knowledge requirements
During the hundreds of mapping we have conducted, we have yet to encounter a cold storage facility that perfectly maintained a determined range under all conditions. Consistently achieving such precise temperature control will, in almost all cases, be unattainable because of the opening of doors, the movement of products in and out, staff entering and exiting, etc.
In other words, even though you define your low and high values, there will be momentary excursions to these limits.
As a result, you also need a level of knowledge about your product’s tolerance.
What you need to know When the conditions go outside your high and low limits, it is necessary to understand and be aligned on the following:
- The accepted time frame: For how long can we accept excursions outside the predefined limits?
- The extent: To what degree can the temperature exceed the limits?
- The variations: Do these vary for different places in the cold chain and for different product types?
Many auditors will expect to see a comprehensive risk assessment that explains the basis for determining that stored products can tolerate these momentary excursions.
Tier 3: Operational knowledge
As the top tier of insights into the requirements, operational knowledge is an understanding of your facility's temperature control capabilities derived from hands-on experience.
In accordance with the basic quality principle of “continuous improvement”, gathering indicators of your facility’s performance (and non-performance) under certain conditions can form a baseline for defining how homogeneous and stable the temperature should be under different conditions.
What are the differences between “stability” and “homogeneity” when it comes to temperature conditions?
- Stability: Stability refers to the consistency of temperature over time within a facility. This could be over short periods, such as hours, or extend to days or weeks, and even be influenced by seasonal changes. Stability is indicative of how well a facility’s temperature control systems are calibrated and functioning. A stable environment means that the temperatures are reliably maintained within set thresholds, regardless of external temperature fluctuations or operational dynamics like door openings and product movement. This aspect is crucial for products that are sensitive to even minor deviations in temperature, as it ensures that they are stored under continuous, controlled conditions.
- Homogeneity: Homogeneity relates to the even distribution of temperatures throughout a facility. This includes analyzing temperature variations not just along the floor area (length and width) of, for instance, a warehouse but also vertically (height). Good homogeneity means that temperature readings from various points within the storage area are closely grouped and do not vary significantly. This uniformity is vital for ensuring that all stored products, regardless of their specific location within the facility, are subjected to the same conditions. Factors like insulation performance and air circulation play significant roles in achieving high homogeneity. Achieving and maintaining homogeneity can significantly impact an auditor's assessment of a facility’s operational quality.
Action
Make a risk assessment to define:
- Your high- and low-temperature value limits
- The extent these can be exceeded for the specific product and at different stages in the cold chain to still maintain product integrity
- The required levels of stability and homogeneity for temperature conditions in your unit or facility
2: Develop a clear and effective mapping protocol
Regardless of the guidelines you are following, a successful temperature mapping exercise begins with a robust protocol.
Action
Develop a detailed, well-thought-out protocol that clearly outlines elements like:
3: Document from the start (and never stop)
Effective documentation is a cornerstone of the WHO's temperature mapping guidelines, which emphasize the necessity of maintaining detailed records throughout the mapping process.
Starting from the planning phase, articulate the purpose, goals, and expected outcomes of the exercise. Also, make sure to indicate in your protocol how you plan to document. Documentation should include calibration data, temperature logs, and methods for addressing any non-conformities or deviations that may arise.
Also, make sure to document everything that happens during the study. Why did this excursion happen? What is the reasoning behind this? Note anything that will support you in reporting and justifying deviations.
But don’t only rely on WHO
Although WHO is a key entity in the world of compliance standards, and its guidelines provide a solid baseline, you should not rely on them in isolation when it comes to defining your documentation practice. Also, take specific documentation standards into account, such as good documentation practices (CDocP) and the ALCOA+ principles – and, of course, consider documentation requirements in your current SOPs.
Actions:
- Make sure to have proper documentation practices in place before you start the mapping process.
- Provide training for all relevant staff in the defined documentation practices.
- Never stop documenting; write it down every time you encounter something abnormal during the study.

4: Make sure your devices are calibrated
Calibration is a fundamental aspect of the WHO's mapping guidelines. The aim is to ensure that the data loggers (or “Electronic data logging monitor (EDLM)” as it is referred to in the guidelines) you use for the process deliver accurate and reliable data.
According to the guidelines, each logger must come with a NIST-traceable 3-point calibration certificate, maintaining an error margin no greater than ±0.5 °C at each calibration point. For some organizations, ISO 17025-accredited calibration is necessary.
This precise calibration confirms that devices are suitable for the specific conditions of the mapping exercise and capable of recording the full range of expected temperatures, from as low as −30 °C to as high as +60 °C.
Action
Make sure your chosen data loggers are calibrated to your required standards and include valid calibration certificates in the mapping report.

5: Find the right mapping data loggers
Besides calibration, the guidelines also specify that the logger should have programmable data sampling periods, adequate memory for the study's duration, and the ability to download data for analysis.
Action
Before starting a mapping study, select data loggers that meet these criteria.
Also read: How to choose the right temperature mapping equipment

Data loggers (and other tools) built for GxP temperature mapping
Reduce risks and secure a reliable, efficient result with wireless data loggers and full mapping kits (data loggers, software access, and everything else you need) built for GxP. See how it works in our mapping catalog.
6: Conduct “worst case scenario” mappings
“If storage areas are affected by seasonal temperature variations, at least two temperature-mapping studies may be needed in each area to observe the effect of seasonal variation.” (WHO, Supplement 8, “Temperature mapping of storage areas”, 2015).
One of the most common questions we receive is: “Do we have to perform summer and winter mappings?”
To some extent, the WHO is both clear and unclear on this topic. When inspected, WHO states that temperature validation is needed, not necessarily during these seasons, but in “worst-case scenarios.”
In other words, there is a need for worst-case analysis, where you try to affect the equipment or the process in the worst cases.
Does “worst case” always mean “seasonal” when it comes to mappings?
Although there are scenarios where the “worst case” is not summer and winter, it will often be the case for warehouses and other units and facilities affected by outside temperatures, also through the performance of the HVAC system. However, according to WHO, two-season mapping is normally not needed for cold rooms and freezer rooms.
Action: If your unit or facility is exposed to seasonal changes, conduct mapping exercises in the hottest and coldest months to ensure that all fluctuations are monitored correctly and that you have a proper risk assessment plan in place.
7: Develop a comprehensive mapping report
Once data collection is complete, the next phase is analysis and reporting. The data should be reviewed against the defined criteria to identify any deviations or areas of concern.
Action
Your final report should include:
- Detailed data analysis comparing actual conditions against expected outcomes.
- Identification of any inconsistencies or anomalies.
- Recommendations for corrective actions and improvements.
Download a full mapping report template here:


Temperature mapping for GxP guide
Get a detailed step-by-step guide for planning and carrying out efficient temperature mapping studies in GxP environments.
How do the WHO mapping guidelines align with other guidelines?
As the WHO’s mapping approach presents the best practices for the process, it is no surprise that there is a red thread throughout this and other guidelines.
The WHO’s guidelines are designed to be inherently compatible with a variety of other standards, ensuring a cohesive framework for global compliance. Some of these offer more extensive coverage on certain topics, such as risk assessments and documentation. For instance:
- the ISPE’s guidelines for temperature control chamber mapping and monitoring are highly recommended for their depth.
- the PIC/S guidelines delve deeply into various complexities.
Essentially, the WHO guidelines provide a foundational baseline upon which you can build more specific guidelines if needed.
Besides the more extensive guidelines, the WHO’s guidelines are aligned with and, to some extent, serve as an expansion on, for instance, GxP principles, The United States Pharmacopeia (USP) <1079>, which are usually used in Good Storage Practices and Distribution Practices, as well as The International Conference on Harmonisation’s (ICH) approaches.
Key takeaways:
- WHO guidelines are a good place to start, aligned with other international and national guidelines, and you will do nothing wrong by following them.
- If you need more detailed information or guidance on certain topics, you can, for instance look to the ISPE or PIC/S.
Summing up – why should you follow the WHO temperature mapping guidelines?
In conclusion, WHO guidelines are integral to maintaining high standards in GxP environments. They provide a trusted framework that not only supports compliance but also drives quality improvement and efficiency. By aligning with WHO standards, you can ensure that your temperature mapping practices robustly support the overall goal of product integrity and patient safety.
Also read: 9 questions: Ensuring 21 CFR Part 11-reliable temperature compliance in pharma
Learn more
Eupry’s temperature mapping solutions are built upon WHO’s and other key industry standards to provide efficient, reliable thermal validations in GxP.
Sources
- https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/distribution/trs961-annex9-supp8.pdf
- https://iris.who.int/bitstream/handle/10665/352894/9789240042773-eng.pdf?sequence=1


