Automated temperature monitoring for GxP
- without calibration headaches
- Automated calibration: No downtime or worries with on-the-wall calibration done in seconds.
- Audit reports in 3 clicks: Full, digital audit reports across TCUs, facilities, and departments.
- Live monitoring on any screen: Wireless data loggers, instant alerts, and real-time insigths.
Instant access to all technical specifications, solution options, and more.

Trusted by +1000 GxO companies worldwide
Temperature monitoring shouldn’t be (this) hard
Manual data collection
Manually handling data transfer, monitoring, deviations, calibrations, and reporting eats up resources that could - and should - be used for more valuable work.
Delayed deviation detection
Misaligned, paper-based, and delayed processes lead to errors, traceability gaps, and increased risk of – and worries about – non-compliance.
Complex, costly setups
Inconsistent processes across sites and multiple vendors for monitoring, calibration, and validation drive up complexity and costs - and stand in the way of scaling.
Lack of visibility
Varying processes and no live insights into facility performance and across sites lead to compliance issues, redundant work, and stressful audits.
Free time and minimize risks with automated, wireless monitoring
- Automated monitoring and deviation handling
- 3-click audit reports
- On-the-wall calibration in less than 10 seconds
All the quality, none of the chaos.

Stay compliant, always
Be in full control of compliance at all times and showcase top-tier quality with live monitoring, instant alerts, and no manual errors, process gaps, or missed quality issues.
Free up (a lot of) time
Fewer disruptions, smaller workload, no paperwork. Spend way (!) less time on compliance with automation and one easy-to-use digital solution gathering all temperature compliance.
Get full visibility - live
Get one digital overview of the quality of your entire operation – and showcase it easily to auditors. No unspotted issues or digging through papers, just stable compliance.
Minimize costs
Cut overall costs and avoid surprise fees with a unified, cost-optimized solution that includes calibration and a full warranty. No hardware issues, no replacement costs.

High compliance rating by Novo Nordisk
Eupry Aps provided (…) documentation to conclude that procedures and efficient controls to ensure compliance are in place and well-functioning as intended in all key areas/processes.
ISO AUDIT REPORT by Novo Nordisk, June 2023

Wireless data loggers built for GMP and GDP
The Wi-Fi-based data loggers automatically send data through Wi-Fi to your (included) platform and let you monitor conditions live.
- No manual data transfers or alarm logging
- Live monitoring of temperature, humidity, and similar
- Works with any TCU or facility – from fridge to warehouse
- Validated backups ensure data integrity (even if Wi-Fi is out)

Monitoring app = one platform, all your data, and reporting in 3 clicks
Track compliance on any screen – from your desktop to your phone.
The monitoring platform gives you a full overview across units and facilities.
It holds everything you need to secure and demonstrate compliance: audit reports, monitoring data, deviation logs, calibration certificates, etc. – all the historical data your auditors expect, just a few clicks away.
No spreadsheets. No paperwork. No digging for data.
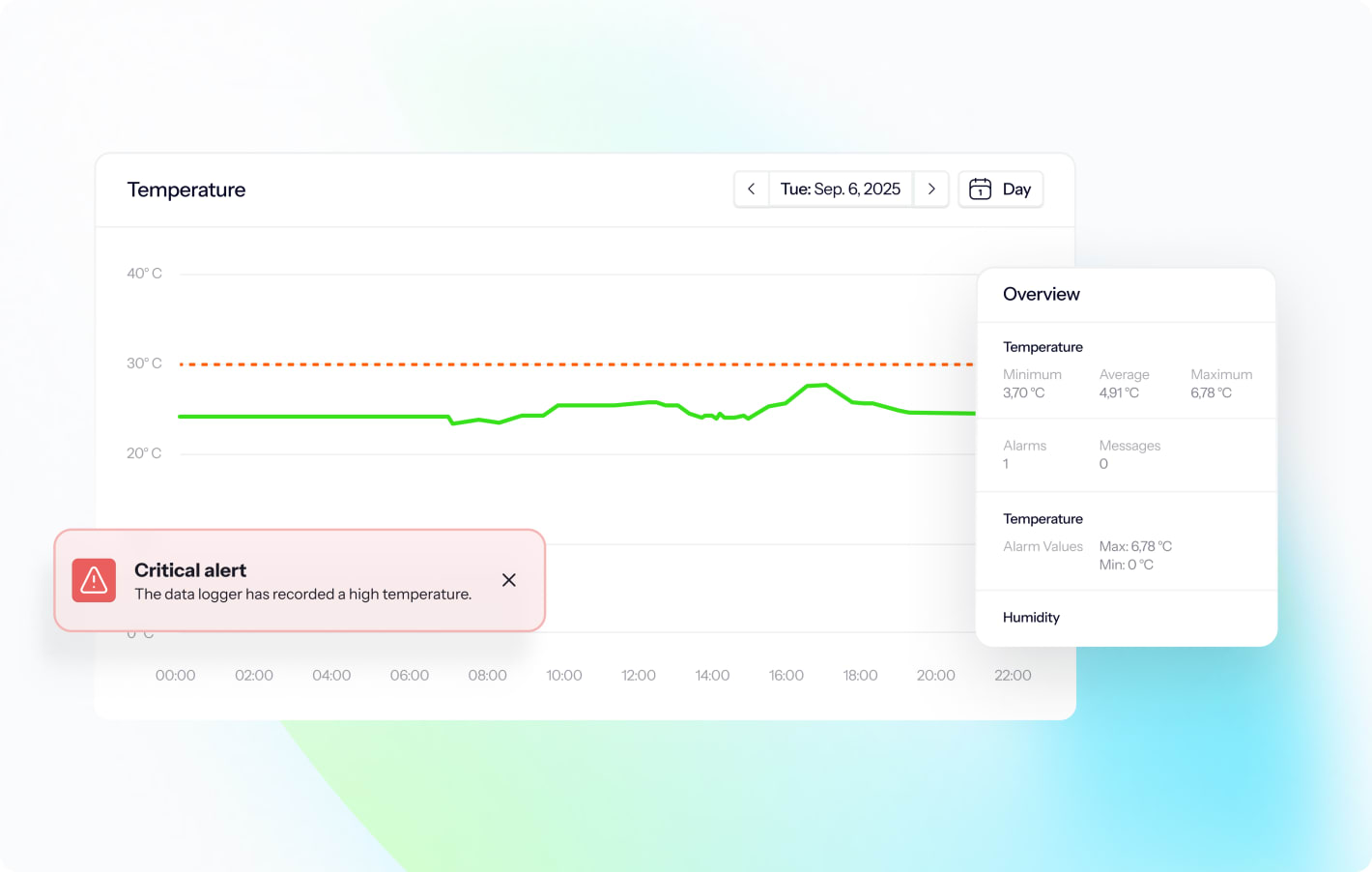
Instant deviation alerts
Make sure nothing critical goes unnoticed. Handle deviations fast and compliantly with instant alarms and automated deviation management.
- Reduce alarms: Filter out insignificant alarms and grey zones.
- Act faster: Get instant alarms on SMS and email.
- Log easier: Alarms are automatically logged to easily create NCRs.
- Stay in control: Personalize who gets what alerts and when.
Even small fluctuations in temperatures can have an impact on the quality of our samples. Eupry gives us trustworthy insights into this.
Jennifer Bintz, Research Technician at InProTher

Included calibration in a click
Temperature monitoring with on-the-wall calibration included – reduce calibration time by 95% and eliminate downtime.
When it is calibration time, we simply send you newly calibrated tips, and, instead of replacing the whole logger, you just click a new tip on each device – a few seconds and calibration is done.


Temperature mapping services - if you need it
Need to peform mapping too? No worries! We also offer GxP mapping solutions - built into the same solution using the same equipment.
- A solution that fits you: From “do it yourself”-kit or our specialist handling it all for you.
- Digital mappings: Save time and reduce risks with automated data capture and digital reports.
- Specialists on call: With hundreds of mappings behind us, we understand YOUR requirements.


Download a temperature monitoring catalog
Prevent mistakes and stop the hunt for data with automated temperature monitoring built for GMP, GDP, and other GxP industries. Get instant access to all the details and see how it works our catalog.
Trusted by +1.000 companies worldwide
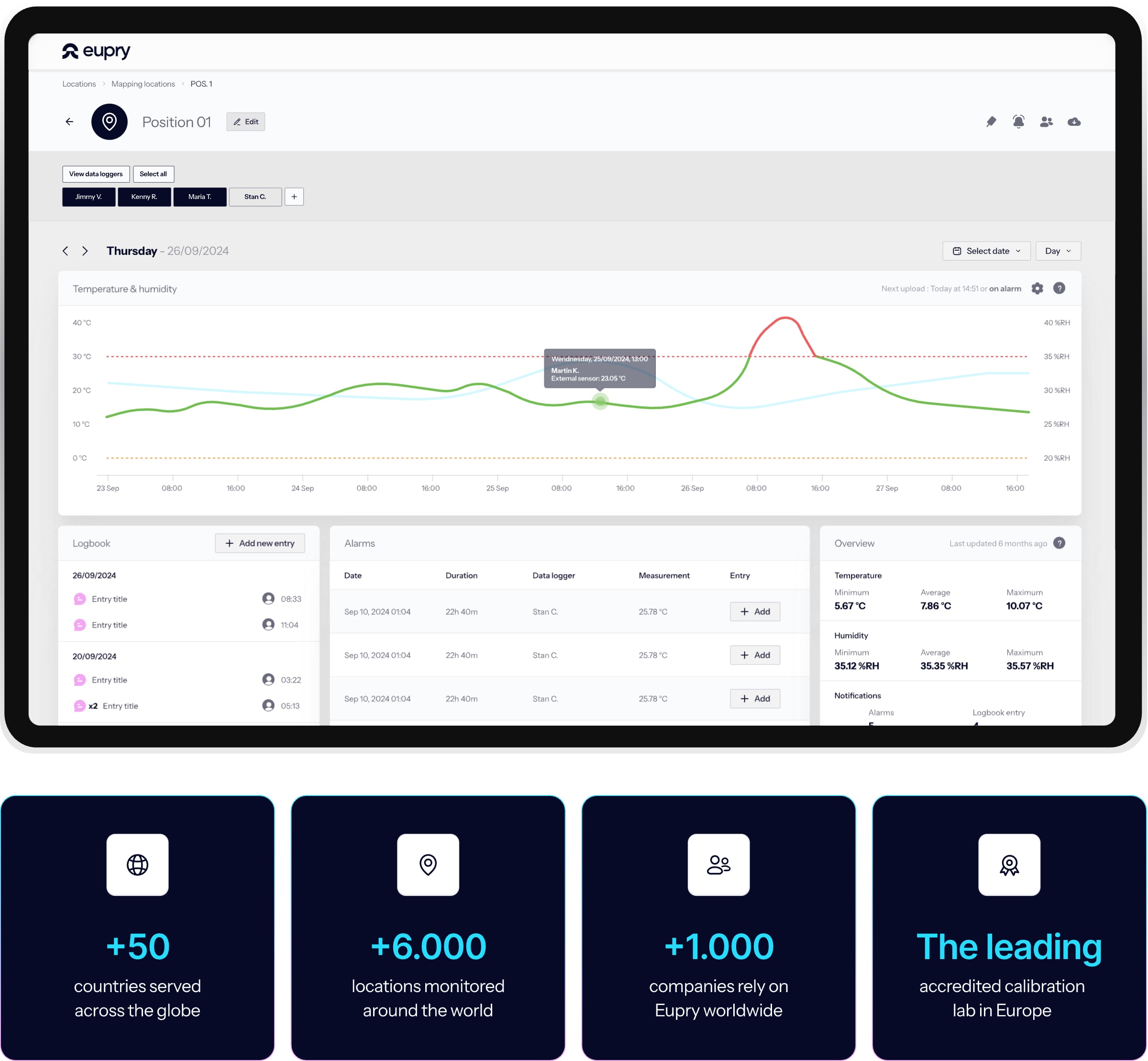
All sites, all compliance issues, ONE dashboard
How do you know which sites have compliance issues right now? The Compliance Status Dashboard shows every alarm and upcoming calibration across your operation – in one view.
Quality support - on your terms
Temperature compliance professionals sit in the ”hot seat”. That is why we are there to support you at every step, if you need us.
The solution gives you 100% control of your monitoring efforts, but should you need assistance, our team is more than happy to support you – all day, all week.

All your compliance boxes? Consider them ticked
21 CFR Part 11 module
The system has a specialized FDA 21 CFR Part 11 module, which makes it simple for you and your team to comply with the regulations.
ISO 17025-accreditation
Accredited or traceable/NIST calibration without downtime is included in the service. No extra loggers, no downtime, and no fragmented data.
ISO 27001-certification
Eupry is ISO 27001 certified, which means our system and processes are built to keep your data safe and compliant. All data is encrypted using AES and stored with continual backup procedures for as long as you need.
Growing with regulations
We stay on top of compliance (so you do not have to). The solution makes worrying about changes in the compliance landscape a thing of the past.
ISO 9001-certified quality
Our ISO 9001 certification means processes and services always meet the highest quality benchmarks, ensuring efficiency and reliability.
IT and hardware validated
Software and hardware are fully validated to meet industry requirements and reduce the risk of costly errors or data integrity issues.
IQ OQ Qualification protocol
Achieve compliance through the included “Qualification Protocol” to ensure proper installation, correct setting configuration, and effective deviation management.
Safe storage of personal data
Personal data is handled according to CCPA and GDPR standards, providing an extra layer of assurance for your data's security.
The Eupry Trust Hub
All compliance documentation in one place
We understand that vetting a new GxP partner involves a lot of questions. The Trust Hub is designed to make that process faster and easier. It gathers all the information you need to assess our compliance and processes – from accreditations, certifications, and quality management approach to how we handle data security.
We have saved around 50-70% of the time we spent on temperature monitoring.
Anders Rasmussen, Senior Logistic Manager at Dechra Pharmaceuticals PLC
How the temperature monitoring solution works
Eupry provides Wi-Fi-based temperature monitoring, on-the-wall calibration, and mapping in one connected, automated, and accredited solution.
Temperature monitoring built for your industry
Eupry's wireless compliance solution is meticulously designed to meet the unique and ever-evolving temperature monitoring needs of GxP industries.
Tip! Looking for other industries? Eupry's solutions is also useful for other GxP-regulated industries. Contact us to learn more.
Temperature monitoring for healthcare logistics
The solution is designed for the specific challenges of temperature monitoring in warehouses and other logistics environments – from managing multiple sites and diverse goods to maintaining robust wireless connectivity and seamless data integration across large storage areas.
Robust for large facilities
Wireless setup, Wi-Fi-based data loggers, and robust connectivity mean that the solution easily accommodates large spaces.
Tailored support
Our compliance specialists can help you define everything from the optimal setup based on your specific floor plans to the diverse needs of different products.
Remote access
No need to access individual devices, which can be pretty neat in a big storage space, and data can be accessed from anywhere at any time.
GDP compliance
The system is fully aligned with industry standards such as the FDA’s enforcement of GDP, and compliance levels are flexible catering to various goods and helping to control costs.
Temperature monitoring for pharma
Eupry's solution addresses the stringent regulatory demands of the pharmaceutical industry.
Simple, digital audit processes
Digital tools to export audit-ready reports with a few clicks.
Compliance in the forefront
ISO 17025-accredited calibration, ISO 9001 accreditation, ISO 27001 security, and much more.
Continuous data integrity
Reliable backup systems, secure storage, user access control, and much more.
From lab settings to mass production
Adapting to the needs of the entire pharmaceutical value chain – from R&D to GMP and GDP.
Temperature monitoring for biotech
In the fast-paced world of biotech, the system offers flexibility and precision.
Accuracy
Accuracy: High-precision sensors support good laboratory practice (GLP) with accredited calibration.
Simplifying auditing
Robust deviation management and reporting tools simplify the audit process.
Scalability
The subscription model, easy installation, and flexible wireless setup accommodate the growth of many biotech operations.
Automated data recording
Ensuring data integrity and real-time alerts.
Client Testimonials

Talk to a monitoring specialist
Need a quote or more information? We are here to help. Temperature monitoring and compliance is what we do, and we know how to turn your requirements into action and can help uncover your needs; from quick questions to complex projects.
Let us know what you are looking for, and we will send the information – or set up a talk.












